Load-Complexity Categories: Simple, Moderate A, Moderate B, and Complex (Torsion/Shear)
The general theme of this section is that regional variations in predominant collagen fiber orientation (CFO) coupled with in vivo strain data suggest that habitual loading of a bone or bone region can be best understood in the context of one of four “load-complexity categories” (Fig. 1). In order to understand the rationale for the criteria used to create the four load-complexity categories, it is useful to start by distinguishing the load histories that are at the extremes — simple bending (least complex) to torsion combined with bending and compression (most complex). Another way to think about these extremes is that there is greater “load predictability” in simple bending and less “load predictability” in complex torsion/bending. Figure 2 shows examples of diaphyseal regions of some bones that can be placed in one of the four load-complexity categories. These four categories are based on the three general/habitual load categories that have been described by Currey (1984), which represent a spectrum from relatively simple to complex loading, respectively: A) short cantilever (e.g., sheep, deer, and equine calcanei), B) curved column (e.g., equine and sheep radii), and C) straight or quasi-curved column (e.g., equine third metacarpals and sheep tibiae). As shown in figure 1, the four load-complexity categories are based on typical ranges of neutral axis rotation; where the neutral axis rotates the most there is prevalent/predominant torsion (the highest-complexity load category). In turn, they help to conceptualize and simplify the characteristic loading patterns of diaphyseal regions where a focus on structural characteristics can be misleading. Additional studies are needed to develop more rigorous, and empirically tested, criteria for distinguishing these categories (see Moreno et al. (2008)).
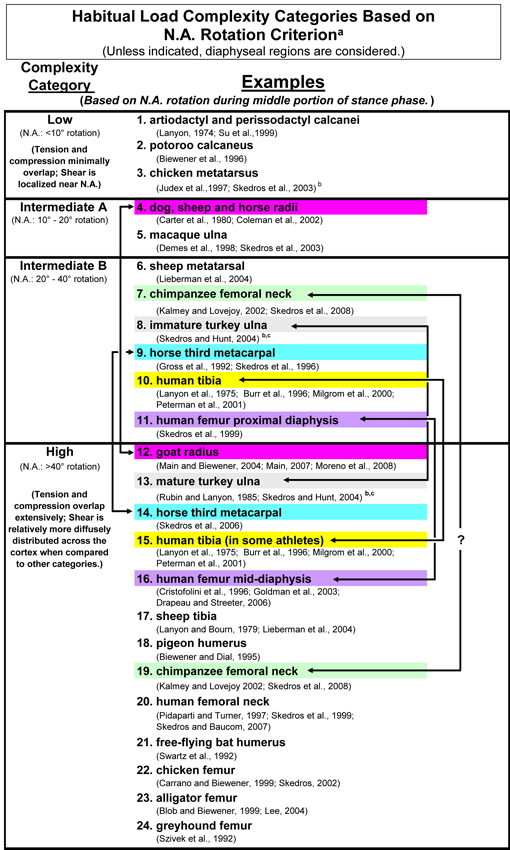

Fig. 1 The four load-complexity categories. Studies that either support segregating these bones or bone regions into these categories or support the comments in the footnote of this table are listed here (Biewener 1990; Biewener et al. 1996; Burr et al. 1996; Carter et al. 1980; Coleman et al. 2002; Currey and Alexander 1985; Demes et al. 1998; Gross et al. 1992; Kalmey and Lovejoy 2002; Lanyon 1974; Lanyon et al. 1975; Lieberman et al. 2004; Main 2007; Main and Biewener 2004; Milgrom et al. 2000; Moreno et al. 2008; Pearson and Lieberman 2004; Peterman et al. 2001; Rubin et al. 1996; Rubin and Lanyon 1985; Ruff et al. 2006; Skedros et al. 2008; Skedros et al. 2003a; Skedros and Hunt 2004; Skedros et al. 2004; Skedros et al. 1996; Su et al. 1999; Turner 1998)
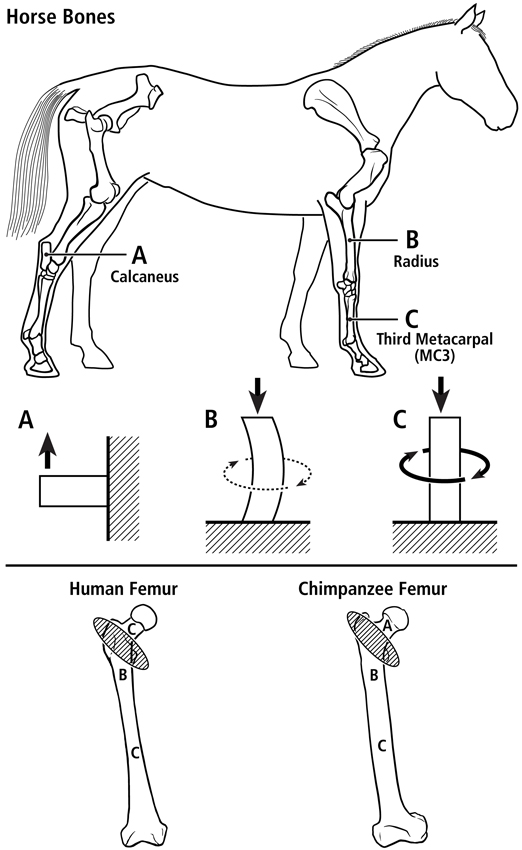
Fig. 2 At top are lateral-to-medial views of the right forelimb and hindlimb skeletons of an adult horse showing a spectrum from simple loading to complex loading, respectively: calcaneus (A), radius (B), and third metacarpal (MC3) (C). The drawings below are simplified renditions of each bone type, showing: A) the calcaneus as a cantilevered beam, B) the radius as a curved beam with longitudinal loading; the curvature accentuates bending. Torsion (dotted line) is also present but is less than the torsion in the MC3 (solid circular line in C), and C) the MC3 with off-axis longitudinal loading producing bending and torsion, the latter being greater than in the other two bones. Several studies reporting in vivo strain data were used to create these drawings (Biewener et al. 1983a; Biewener et al. 1983b; Gross et al. 1992; Lanyon 1974; Rubin and Lanyon 1982; Schneider et al. 1982; Turner et al. 1975).
At bottom are the hypothesized multi-domains for a human femur (left) and chimpanzee femur (right); the letters within the femora correspond to the basic load conditions in the horse bones. The intertrochanteric regions of these bones (indicated by ellipses with oblique lines) are transition zones between the neck and proximal diaphyseal domains. In accordance with predominant CFO data (Beckstrom et al. 2010; Skedros et al. 1999b), these drawings show the speculation that the chimpanzee femoral neck (also more robust and elliptical in cross-sectional shape) receives more prevalent/predominant bending than the human femoral neck.
Bending vs. torsion.
Becoming proficient at differentiating general features of bone morphology (from a bone’s shape and girth to its microstructure/nanostructure) in habitual bending vs. habitual torsion will help to increase your awareness of the strengths and weaknesses of using histological organization to infer the load history of a bone or bone region. The assertion that predominant bending or torsion are the most representative load histories of bone diaphyses, with most other load regimes being somewhere along the continuum between these, is supported by in vivo strain measurements from bones of various mammalian and avian species that typically demonstrate these load regimes as being most prevalent during peak loading of controlled ambulation (Lanyon and Bourn, 1979; Lieberman et al., 2003; de Margerie et al., 2005). In fact, bending is the load regime that is most highly conserved in many appendicular long bone diaphyses (Rubin and Lanyon, 1984), and bending also produces the majority (>70%) of longitudinal strains occurring during peak loading of controlled in vivo activity (Biewener et al., 1986; Biewener and Bertram, 1993). The bending vs. torsion distinction is also clinically relevant and is related to regional patterns of predominant CFO and distributions of osteon morphotypes. For example, bone failure at the material level directly influences structural failure of the whole bone, and this is especially true in bending- and torsion-related fracture (Ebacher et al., 2007).
When you think of bending think of roughly discrete regions of tension, compression, and shear.
Bending creates a neutral axis, which is a region where longitudinal strains (i.e., along the long axis of the bone) drop to zero in materials that are ideal (isotropic, uniform, and continuous). In other words, the neutral axis is the location (or a “zone” in typical bones because the neutral axis is never stationary) where there is a transition from longitudinal tension to compression. In the neutral axis regions, however, shear strains become large because the maximum strains are obliquely oriented with respect to the long axis of the bone. Although longitudinal strains drop toward zero in neutral axis regions, strain gradients and fluid flux increase — these parameters influence nutrient delivery (Judex et al., 1997). Bending, therefore, creates a dilemma because the mechanical properties, including fracture and microdamage mechanics, of cortical bone differ in tension, compression, and shear (mechanical properties of bone in tension and shear are worse than compression) (see section on the “Shear Resistance-Priority Hypothesis”). Differential histological modifications are necessary in order to more optimally resist and/or accommodate the formation of fatigue microdamage in these common non-uniform, potentially deleterious, strain environments that are engendered by habitual bending (Reilly et al., 1997; Akkus et al., 2003). To reduce its tendency to fracture in regions where physiologic loading produces a degradation of mechanical integrity, regional variations in bone nanostructure (e.g., CFO and collagen molecular cross-links) and microstructure (e.g., osteon morphotypes, osteon density, percent osteon area, and osteon diameter) can modify matrix mechanical properties (e.g., strength, fatigue resistance, and energy absorbed to failure) (Evans and Bang, 1966, 1967; Evans and Vincentelli, 1974; Simkin and Robin, 1974; Carter et al., 1976; Evans, 1976; Moyle et al., 1978; Vincentelli and Grigorov, 1985; Corondan and Haworth, 1986; Yeni et al., 1997; Hiller et al., 2003; Skedros et al., 2006; Diab and Vashishth, 2007).
When you think of torsion think of diffusely distributed shear.
In contrast to habitual bending, if a bone is loaded primarily in torsion, regional variations in nanostructural and microstructural adaptations (Table 1) would not be expected because there are typically no significant temporal/spatial disparities in strain modes. As noted above, the prevalent mode in torsion is shear, and the general/diffuse distribution of this strain mode does not warrant regional histological adaptation (Skedros and Hunt, 2004; Skedros et al., 2011; Skedros et al., 2013).

Table 1 Structural and material characteristics of a limb-bone diaphysis (reference : (Barth et al. 2010))
References
References
Akkus O, Knott DF, Jepsen KJ, Davy DT, Rimnac CM. 2003. Relationship between damage accumulation and mechanical property degradation in cortical bone: Microcrack orientation is important. J Biomed Mater Res 65A:482-488.
Barth HD, Launey ME, Macdowell AA, Ager JW, 3rd, Ritchie RO. 2010. On the effect of X-ray irradiation on the deformation and fracture behavior of human cortical bone. Bone 46:1475-1485.
Beckstrom A, Skedros J, Kiser C, Keenan K. 2010. Predominant collagen fiber orientation data support the multi-domain load hypothesis in the chimpanzee femur. Am J Phys Anthropol Supplement 50:63.
Biewener AA. 1990. Biomechanics of mammalian terrestrial locomotion. Science 250:1097-1103.
Biewener AA, Bertram JEA. 1993. Mechanical loading and bone growth in vivo. In: Hall BK, editor. Bone, Volume 7, Bone Growth – B. Boca Raton, FL: CRC Press. p 1-36.
Biewener AA, Fazzalari NL, Konieczynski DD, Baudinette RV. 1996. Adaptive changes in trabecular architecture in relation to functional strain patterns and disuse. Bone 19:1-8.
Biewener AA, Swartz SM, Bertram JEA. 1986. Bone modeling during growth: Dynamic strain equilibrium in the chick tibiotarsus. Calcif Tissue Int 39:390-395.
Biewener AA, Thomason J, Goodship A, Lanyon LE. 1983a. Bone stress in the horse forelimb during locomotion at different gaits: A comparison of two experimental methods. J Biomech 16:565-576.
Biewener AA, Thomason J, Lanyon LE. 1983b. Mechanics of locomotion and jumping in the forelimb of the horse (Equus): In vivo stress developed in the radius and metacarpus. J. Zool. Lond. 201:67-82.
Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. 1996. In vivo measurement of human tibial strains during vigorous activity. Bone 18:405-410.
Carter DR, Hayes WC, Schurman DJ. 1976. Fatigue life of compact bone-II. Effects of microstructure and density. J. Biomech. 9:211-218.
Carter DR, Smith DJ, Spengler DM, Daly CH, Frankel VH. 1980. Measurements and analysis of in vivo bone strains on the canine radius and ulna. J. Biomech. 13:27-38.
Coleman JC, Hart RT, Owan I, Takano Y, Burr DB. 2002. Characterization of dynamic three-dimensional strain fields in the canine radius. J Biomech 35:1677-1683.
Corondan G, Haworth WL. 1986. A fractographic study of human long bone. J Biomech 19:207-218.
Currey JD. 1984. Can strains give adequate information for adaptive bone remodeling? Calcif Tissue Int 36:S118-S122.
Currey JD, Alexander RM. 1985. The thickness of the walls of tubular bones. J Zool Lond 206:453-468.
de Margerie E, Sanchez S, Cubo J, Castanet J. 2005. Torsional resistance as a principal component of the structural design of long bones: comparative multivariate evidence in birds. Anat Rec A Discov Mol Cell Evol Biol 282:49-66.
Demes B, Stern JT, Jr., Hausman MR, Larson SG, McLeod KJ, Rubin CT. 1998. Patterns of strain in the macaque ulna during functional activity. Am J Phys Anthropol 106:87-100.
Diab T, Vashishth D. 2007. Morphology, localization and accumulation of in vivo microdamage in human cortical bone. Bone 40:612-618.
Ebacher V, Tang C, McKay H, Oxland TR, Guy P, Wang R. 2007. Strain redistribution and cracking behavior of human bone during bending. Bone 40:1265-1275.
Evans FG. 1976. Mechanical properties and histology of cortical bone from younger and older men. Anatomical Record 185:1-12.
Evans FG, Bang S. 1966. Physical and histological differences between human fibular and femoral compact bone. In: Evans FG, editor. Studies on the anatomy and function of bone and joints. New York: Springer. p 142-155.
Evans FG, Bang S. 1967. Differences and relationships between the physical properties and the microscopic structure of human femoral, tibial and fibular cortical bone. Am J Anat 120:79-88.
Evans FG, Vincentelli R. 1974. Relations of the compressive properties of human cortical bone to histological structure and calcification. J Biomech 7:1-10.
Gross TS, McLeod KJ, Rubin CT. 1992. Characterizing bone strain distribution in vivo using three triple rosette strain gauges. J. Biomech. 25:1081-1087.
Hiller LP, Stover SM, Gibson VA, Gibeling JC, Prater CS, Hazelwood SJ, Yeh OC, Martin RB. 2003. Osteon pullout in the equine third metacarpal bone: Effects of ex vivo fatigue. J Orthop Res 21:481-488.
Judex S, Gross TS, Zernicke RF. 1997. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in adult skeleton. J Bone Miner Res 12:1737-1745.
Kalmey JK, Lovejoy CO. 2002. Collagen fiber orientation in the femoral necks of apes and humans: Do their histological structures reflect differences in locomotor loading? Bone 31:327-332.
Lanyon LE. 1974. Experimental support for the trajectorial theory of bone structure. J Bone Joint Surg 56B:160-166.
Lanyon LE, Bourn S. 1979. The influence of mechanical function on the development and remodeling of the tibia: An experimental study in sheep. J Bone Joint Surg 61-A:263-273.
Lanyon LE, Hampson WG, Goodship AE, Shah JS. 1975. Bone deformation recorded in vivo from strain gauges attached to the human tibial shaft. Acta Orthop Scand 46:256-268.
Lieberman DE, Pearson OM, Polk JD, Demes B, Crompton AW. 2003. Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J Exp Biol 206:3125-3138.
Lieberman DE, Polk JD, Demes B. 2004. Predicting long bone loading from cross-sectional geometry. Am J Phys Anthropol 123:156-171.
Main RP. 2007. Ontogenetic relationships between in vivo strain environment, bone histomorphometry and growth in the goat radius. J Anat 210:272-293.
Main RP, Biewener AA. 2004. Ontogenetic patterns of limb loading, in vivo bone strains and growth in the goat radius. J Exp Biol 207:2577-2588.
Milgrom C, Finestone A, Simkin A, Ekenman I, Mendelson S, Millgram M, Nyska M, Larsson E, Burr D. 2000. In-vivo strain measurements to evaluate the strengthening potential of exercises on the tibial bone. J Bone Joint Surg Br 82:591-594.
Moreno CA, Main RP, Biewener AA. 2008. Variability in forelimb bone strains during non-steady locomotor activities in goats. J Exp Biol 211:1148-1162.
Moyle DD, Welborn JW, 3rd, Cooke FW. 1978. Work to fracture of canine femoral bone. J Biomech 11:435-440.
Pearson OM, Lieberman DE. 2004. The aging of Wolff’s law: Ontogeny and responses to mechanical loading in cortical bone. Yearbook of Physical Anthropology 47:63-99.
Peterman MM, Hamel AJ, Cavanagh PR, Piazza SJ, Sharkey NA. 2001. In vitro modeling of human tibial strains during exercise in micro-gravity. J Biomech 34:693-698.
Reilly GC, Currey JD, Goodship AE. 1997. Exercise of young thoroughbred horses increases impact strength of the third metacarpal bone. J Orthop Res 15:862-868.
Rubin CT, Gross TS, Qin Y, Fritton SP, Guilak F, McLeod KJ. 1996. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J Bone Joint Surg 78A:1523-1533.
Rubin CT, Lanyon LE. 1982. Limb mechanics as a function of speed and gait: A study of functional strains in the radius and tibia of horse and dog. J Exp Biol 101:187-211.
Rubin CT, Lanyon LE. 1984. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol 107:321-327.
Rubin CT, Lanyon LE. 1985. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411-417.
Ruff C, Holt B, Trinkaus E. 2006. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am J Phys Anthropol 129:484-498.
Schneider RK, Milne DW, Gabel AA, Groom JJ, Bramlage LR. 1982. Multidirectional in vivo strain analysis of the equine radius and tibia during dynamic loading with and without a cast. Am J Vet Res 43:1541-1550.
Simkin A, Robin G. 1974. Fracture formation in differing collagen fiber pattern of compact bone. J Biomech 7:183-188.
Skedros JG, Beckstrom AB, Kiser CJ, Bloebaum RD. 2008. The importance of bipedality/bending in mediating morphological adaptation in the chimpanzee femoral neck might be overstated. Amer J of Phys Anthro Suppl 46:195.
Skedros JG, Dayton MR, Sybrowsky CL, Bloebaum RD, Bachus K. 2006. The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. J Exp Biol 209:3025-3042.
Skedros JG, Demes B, Judex S. 2003. Limitations in the use of predominant collagen fiber orientation for inferring loading history in cortical bone. Am J Phys Anthropol Suppl 36:193.
Skedros JG, Hughes PE, Nelson K, Winet H. 1999. Collagen fiber orientation in the proximal femur: Challenging Wolff’s tension/compression interpretation. J Bone Miner Res 14:S441.
Skedros JG, Hunt KJ. 2004. Does the degree of laminarity mediate site-specific differences in collagen fiber orientation in primary bone? An evaluation in the turkey ulna diaphysis. J. Anat. 205:121-134.
Skedros JG, Hunt KJ, Bloebaum RD. 2004. Relationships of loading history and structural and material characteristics of bone: Development of the mule deer calcaneus. J Morphol 259:281-307.
Skedros JG, Keenan KE, Williams TJ, Kiser CJ. 2013. Secondary osteon size and collagen/lamellar organization (“osteon morphotypes”) are not coupled, but potentially adapt independently for local strain mode or magnitude. Journal of Structural Biology 181:95-107.
Skedros JG, Kiser CJ, Keenan KE, Thomas SC. 2011. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat 218:480-499.
Skedros JG, Mason MW, Nelson MC, Bloebaum RD. 1996. Evidence of structural and material adaptation to specific strain features in cortical bone. Anat Rec 246:47-63.
Su SC, Skedros JG, Bachus KN, Bloebaum RD. 1999. Loading conditions and cortical bone construction of an artiodactyl calcaneus. J Exp Biol 202 Pt 22:3239-3254.
Turner AS, Mills EJ, Gabel AA. 1975. In vivo measurement of bone strain in the horse. Am J Vet Res 36:1573-1579.
Turner CH. 1998. Three rules for bone adaptation to mechanical stimuli. Bone 23:399-407.
Vincentelli R, Grigorov M. 1985. The effect of Haversian remodeling on the tensile properties of human cortical bone. J Biomech 18:201-207.
Yeni YN, Brown CU, Wang Z, Norman TL. 1997. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 21:453-459.

