Evidence of different load-complexity categories/domains within the same bone lead to the development of Teambone’s multi-domain load hypothesis. As illustrated in figure 1 (at top), we have applied the concept of distinct load-complexity ‘domains’ to help make sense of differences in structural and material (histomorphological) characteristics, and their potential “interactions”, between different bones in the same animal (Skedros et al., 2009). Figure 1 (at bottom) and figure 2 show how to apply these categories to the hypothesized presence of different load domains within human and chimpanzee femora. In these examples, we argue that the greater and lesser trochanter region (the “intertrochanteric region”) in each bone is a transition zone between the proximal and distal “load domains” (Skedros and Baucom, 2007).
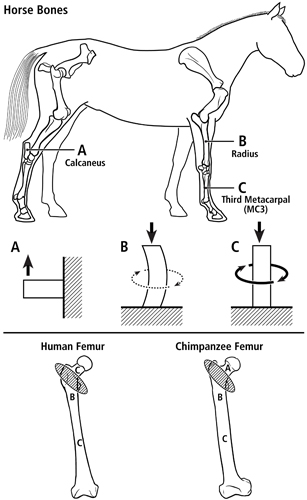
Fig. 1 At top are lateral-to-medial views of the right forelimb and hindlimb skeletons of an adult horse showing a spectrum from simple loading to complex loading, respectively: calcaneus (A), radius (B), and third metacarpal (MC3) (C). The drawings below are simplified renditions of each bone type, showing: A) the calcaneus as a cantilevered beam, B) the radius as a curved beam with longitudinal loading; the curvature accentuates bending. Torsion (dotted line) is also present but is less than the torsion in the MC3 (solid circular line in C), and C) the MC3 with off-axis longitudinal loading producing bending and torsion, the latter being greater than in the other two bones. Several studies reporting in vivo strain data were used to create these drawings (Biewener et al. 1983a; Biewener et al. 1983b; Gross et al. 1992; Lanyon 1974; Rubin and Lanyon 1982; Schneider et al. 1982; Turner et al. 1975).
At bottom are the hypothesized multi-domains for a human femur (left) (also see Fig. 2) and chimpanzee femur (right); the letters within the femora correspond to the basic load conditions in the horse bones. The intertrochanteric regions of these bones (indicated by ellipses with oblique lines) are transition zones between the neck and proximal diaphyseal domains. In accordance with predominant CFO data (Beckstrom et al. 2010; Skedros et al. 1999b), these drawings show the speculation that the chimpanzee femoral neck (also more robust and elliptical in cross-sectional shape) receives more prevalent/predominant bending than the human femoral neck.
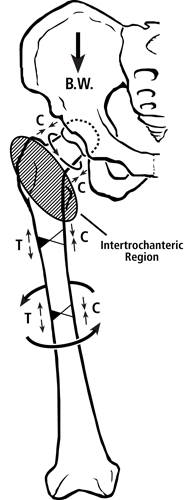
Fig. 2 Habitual loading of a modern human femur showing the multi-domain load hypothesis. Torsion is depicted by the curved lines. B.W. = body weight. In the lower portion of figure 1 are drawings that contrast the hypothesized habitual loadings of the femoral neck domains between chimpanzee and human femora.
In the human femur one of the “proximal domains” — the femoral neck — is likely habitually loaded in net compression by the gluteus medius/minimus and in torsion by the actions of other muscles, body weight, and variable joint reaction forces (Lovejoy, 1988; Skedros and Baucom, 2007) (the femoral head could also be considered a separate “domain” but this possibility is not addressed further here). By contrast, it has been argued that the upper portion of the “distal domain” — the proximal metaphyseal-diaphyseal region — is customarily loaded in bending and torsion (but with relatively less torsion than seen in the mid-neck and mid-diaphysis — discussed below), which is restrained to some extent by the iliotibial band (Skedros and Baucom, 2007). Although the iliotibial band might reduce lateral pitching of the trunk in humans, in vivo strain data obtained during walking at peak loading of stance phase reveal that net tension is still present on the lateral aspect of the proximal metaphysis-diaphysis in the human femur (Aamodt et al., 1997). This study by Aamodt and co-workers is the most important experimental support for considering the human proximal femoral diaphysis as a habitual lateral-medial “tension-compression” environment (Skedros and Baucom, 2007). Quantitative data showing clear patterns of CFO in the sub-trochanteric region and proximal diaphysis of adult human femora are consistent with this interpretation (Skedros et al., 1999; Skedros et al., 2012).
The mid-diaphyseal “load domain” of the human femur.
The mid-diaphyseal load domain extends from the proximal (sub-trochanteric) domain to the distal third of the diaphysis where the strain distribution again changes (Duda et al., 1998). Several studies of CFO patterns in the mid-diaphysis of the human femur have attempted to detect the “expected” history of habitual medial-to-lateral bending (Portigliatti Barbos et al., 1984; Portigliatti Barbos et al., 1987; Goldman et al., 2003). This hypothesized load history is based on the idea that the habitual medial-lateral (compression) to anterior-lateral (tension) bending seen in the proximal diaphysis would be transmitted distally and would be of sufficient intensity/duration to evoke similar regional strain-mode-specific CFO patterns at the mid-diaphysis. However, these studies failed to detect evidence of this load history. Why are these mid-diaphyseal data so different from the proximal diaphyseal data?
Although the strain environment at the mid-diaphyseal femur has, to Teambone’s knowledge, never been measured experimentally in vivo, in vitro strain measurements on femora loaded in simulated single-legged stance contradict the idea of a habitual medial-lateral bending moment at the mid-diaphysis. Results of in vitro strain gauge studies of femora loaded in simulated single-legged stance show both a reduction in the magnitude of the medial-to-lateral bending moment at mid-diaphysis (the bending moment is substantially greater in the sub-trochanteric area) and increased inter-specimen variability of the strain distribution at mid-diaphysis (Oh and Harris, 1978; Cristofolini et al., 1996).
Consequently, the relatively complex load environment of the femoral mid-diaphysis (where torsion > bending) might explain why “expected” tension/compression (lateral/medial) CFO differences are typically absent there, but are present in the proximal diaphysis and subtrochanteric regions (where bending > torsion) (Skedros et al., 1999; Beckstrom et al., 2010; Skedros et al., 2011a; Skedros et al., 2011b). Hence, the “load complexity changes along the femoral diaphysis — from less complex/variable at the proximal diaphysis to more complex/variable at the mid-diaphysis. An explanation for the relatively uniform CFO in adult human mid-diaphyseal femora is similar to that stated in the sections on “Load-Complexity Categories” and the “Shear Resistance-Priority Hypothesis” — bone regions that receive varying amounts of torsion and bending would not be expected to produce clear regional patterns of predominant CFO, and possibly also osteon-related characteristics.
The hypothesized differences in load history from the proximal femoral diaphysis (bending > torsion) to the mid-diaphysis (torsion > bending) in humans are also consistent with the CFO data reported in chimpanzee femora (Beckstrom et al., 2010; Skedros et al., 2011a). This explanation, based on changes in habitual load-complexity, for the dissimilar histological findings between mid- and proximal-diaphyseal chimpanzee femora is also consistent with that offered in a previous study of equine third metacarpals from our laboratory (Skedros et al., 2006) — non-stereotypical and/or complex load histories that produce significant amounts of torsion would not be expected to produce clear regional patterns of CFO-based histological organization (Skedros and Hunt, 2004). Additional support for proximal-distal changes in habitual load complexity within a bone’s diaphysis, and how this could be mediated by strain-related/site-specific differences in thresholds for modeling/remodeling activities, can be found in Hsieh et al. (2001), Skerry (2006), and Espinoza Orías et al. (2009).
It has also been suggested that the relatively circular cross-sectional shape of the human femoral mid-diaphysis reflects cross-sectional morphology expected in a loading environment characterized by prevalent torsion (and the increased variability of the neutral axis location produced by such loading) (Ruff, 1981; Carter and Spengler, 1982; Wainwright et al., 1982). But a quasi circular shape is also typical in the proximal diaphysis where the bending moment is the greatest in the entire femur. Again, this reinforces the idea that cross-sectional shape can be misleading when interpreting load history. These data again support the assertion that patterns of predominant CFO and/or osteon morphotypes are strong predictors of load history because they help distinguish bending from torsional load histories.
References
Aamodt A, Lund-Larsen J, Eine J, Andersen E, Benum P, Schnell Husby O. 1997. In vivo measurements show tensile axial strain in the proximal lateral aspect of the human femur. J Ortho Res 15:927-931.
Beckstrom A, Skedros J, Kiser C, Keenan K. 2010. Predominant collagen fiber orientation data support the multi-domain load hypothesis in the chimpanzee femur. Am J Phys Anthropol Supplement 50:63.
Biewener AA, Thomason J, Goodship A, Lanyon LE. 1983a. Bone stress in the horse forelimb during locomotion at different gaits: A comparison of two experimental methods. J Biomech 16:565-576.
Biewener AA, Thomason J, Lanyon LE. 1983b. Mechanics of locomotion and jumping in the forelimb of the horse (Equus): In vivo stress developed in the radius and metacarpus. J. Zool. Lond. 201:67-82.
Carter DR, Spengler DM. 1982. Biomechanics of fracture. In: Sumner-Smith G, editor. Bone in clinical orthopaedics: A study in comparative osteology. Philadelphia, PA: W.B. Saunders Co. p 305-334.
Cristofolini L, Viceconti M, Cappello A, Toni A. 1996. Mechanical validation of whole bone composite femur models. J Biomech 29:525-535.
Duda GN, Heller M, Albinger J, Schulz O, Schneider E, Claes L. 1998. Influence of muscle forces on femoral strain distribution. J Biomech 31:841-846.
Espinoza Orias AA, Deuerling JM, Landrigan MD, Renaud JE, Roeder RK. 2009. Anatomic variation in the elastic anisotropy of cortical bone tissue in the human femur. J Mech Behav Biomed Mater 2:255-263.
Goldman HM, Bromage TG, Thomas CD, Clement JG. 2003. Preferred collagen fiber orientation in the human mid-shaft femur. Anat Rec 272A:434-445.
Gross TS, McLeod KJ, Rubin CT. 1992. Characterizing bone strain distribution in vivo using three triple rosette strain gauges. J. Biomech. 25:1081-1087.
Hsieh Y-F, Robling AG, Ambrosius WT, Burr DB, Turner CH. 2001. Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J Bone and Min Research 16:2291-2297.
Lanyon LE. 1974. Experimental support for the trajectorial theory of bone structure. J Bone Joint Surg 56B:160-166.
Lovejoy CO. 1988. Evolution of human walking. Sci Am 259:118-125.
Oh I, Harris WH. 1978. Proximal strain distribution in the loaded femur. An in vitro comparison of the distributions in the intact femur and after insertion of different hip-replacement femoral components. J Bone Joint Surg Am 60:75-85.
Portigliatti Barbos M, Bianco P, Ascenzi A, Boyde A. 1984. Collagen orientation in compact bone: II. Distribution of lamellae in the whole of the human femoral shaft with reference to its mechanical properties. Metab Bone Dis and Relat Res 5:309-315.
Portigliatti Barbos M, Carando S, Ascenzi A, Boyde A. 1987. On the structural symmetry of human femurs. Bone 8:165-169.
Rubin CT, Lanyon LE. 1982. Limb mechanics as a function of speed and gait: A study of functional strains in the radius and tibia of horse and dog. J Exp Biol 101:187-211.
Ruff CB. 1981. Structural changes in the lower limb bones with aging at Pecos Pueblo. Philadelphia, PA: University of Pennsylvania.
Schneider RK, Milne DW, Gabel AA, Groom JJ, Bramlage LR. 1982. Multidirectional in vivo strain analysis of the equine radius and tibia during dynamic loading with and without a cast. Am J Vet Res 43:1541-1550.
Skedros J, Hughes P, Nelson K, Winet H. 1999a. Collagen Fiber Orientation in the Proximal Femur: Challenging Wolff’s Tension/Compression Interpretation. In: Journal of Bone and Mineral Research: Twenty-First Annual Meeting of the American Society for Bone and Mineral Research. St. Louis, Missouri.
Skedros JG, Baucom SL. 2007. Mathematical analysis of trabecular ‘trajectories’ in apparent trajectorial structures: the unfortunate historical emphasis on the human proximal femur. J Theor Biol 244:15-45.
Skedros JG, Dayton MR, Sybrowsky CL, Bloebaum RD, Bachus K. 2006. The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. J Exp Biol 209:3025-3042.
Skedros JG, Hughes PE, Nelson K, Winet H. 1999b. Collagen fiber orientation in the proximal femur: Challenging Wolff’s tension/compression interpretation. J Bone Miner Res 14:S441.
Skedros JG, Hunt KJ. 2004. Does the degree of laminarity mediate site-specific differences in collagen fiber orientation in primary bone? An evaluation in the turkey ulna diaphysis. J. Anat. 205:121-134.
Skedros JG, Keenan KE, Halley JA, Knight AN, Bloebaum RD. 2012. Osteon morphotypes and predominant collagen fiber orientation are adaptations for habitual medial-lateral bending in the human proximal diaphysis: Implications for understanding the etiology of atypical fractures. 58th Annual Meeting of the Orthopaedic Research Society 37:1512.
Skedros JG, Kiser CJ, Keenan KE, Samuel CT. 2011a. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat In review.
Skedros JG, Kiser CJ, Mendenhall SD. 2011b. A weighted osteon morphotype score out-performs regional osteon percent prevalence calculations for interpreting cortical bone adaptation. Am J Phys Anthropol 144:41-50.
Skedros JG, Mendenhall SD, Kiser CJ, Winet H. 2009. Interpreting cortical bone adaptation and load history by quantifying osteon morphotypes in circularly polarized light images. Bone 44:392-403.
Skerry TM. 2006. One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J Musculoskelet Neuronal Interact 6:122-127.
Turner AS, Mills EJ, Gabel AA. 1975. In vivo measurement of bone strain in the horse. Am J Vet Res 36:1573-1579.
Wainwright SA, Biggs WD, Currey JD, Gosline JM. 1982. Elements of structural systems. In: Mechanical design in organisms. Princeton, NJ: Princeton University Press. p 254-258.

