It can be argued that many examples of structural/material associations that have been shown in limb-bone diaphyses are circumstantial, bearing little relevance in the context of highly coupled functional adaptation. Additionally, existing knowledge of the capacity for intercellular communication in bone suggests that it is not sufficient for achieving the high level of coordination between modeling and remodeling that would be required to accomplish synergism/compensation in an entire diaphysis or across the breadth of a diaphysis. (This would be especially true with advancing age as osteocytic functions become senescent.) Even in youth, this idea is consistent with data reported by Tommasini et al. (2007), where sexual dimorphism was found to affect tibial size and shape but not tissue-level (remodeling parameters) mechanical properties in a sample of 14 young human tibiae (36.9+8.1 years). However, a rigid/comprehensive refutation of this idea is not wise because structural/material synergism/compensation might be at work to some extent in cases where size-shape relationships occur in a broader size range of individuals of the same species (e.g., Tommasini et al. (2005)), or in cases of precocial vs. altricial limb development and/or ambulation (e.g., artiodactyls, musk oxen, jackrabbits, and seagulls) (Carrier, 1983; Currey and Pond, 1989; Carrier and Leon, 1990; Heinrich et al., 1999).
Results from several of our investigations have also provided us with a basis for arguing that the ultimate goals of modeling and remodeling processes are so distinct they work as if there is a “division of labor” in achieving the goals of bone adaptation (Fig. 1). In this argument, modeling is aimed at influencing whole-bone structural performance; “whole-bone” structural performance typically means that the overall strength and stiffness of the bone when end loads are imparted to it. It is relatively easy to achieve this whole-bone goal by adjusting cortical thickness and/or bone shape.
Modeling:
Modeling activities affect the formation and/or resorption of secondary or non-secondary bone (e.g., primary bone, and trabecular bone in some cases) on periosteal or endosteal surfaces. They are detected as changes and/or differences in a bone’s curvature, cross-sectional shape and/or regional cortical thickness. Consequently, modeling is a concept describing a combination of non-proximate, though coordinated, resorption and formation drifts whose net result is, typically, to change the distribution of bone. Such drifts are called macro-modeling in cortical bone and mini-modeling in cancellous bone. The re-alignment of trabecular tracts along the lines of stress would be a consequence of mini-modeling.
Remodeling:
Remodeling activities affect the replacement of intracortical bone; this is achieved through the activation of basic multicellular units (BMUs = osteoclasts and osteoblasts) that create secondary osteons (Haversian systems) in cortical bone and secondary osteons or hemi-osteons in trabecular bone (Jee et al., 1991; Parfitt et al., 1996). curvature or cross-sectional dimensions) as long as the material is suitable. In fact, if variable adjustments in strain between regions of the same cross-section are signals that reflect the attainment of ‘adapted morphology’ (Kumar et al., 2010), then small adjustments in cortical thickness and/or cross-sectional shape can readily achieve this in large as well as in small volume bones. Achieving this goal by regional material adjustments via remodeling (i.e., osteon formation) is not as efficient or effective. For example, it would not be energetically efficient to modify local strains via osteon-mediated reductions in mineralization and increases in porosity (by increasing the density of osteon central canals). During the attainment of a bone’s adapted morphology (when periosteal/endosteal bone apposition/resorption is still possible) (Fig. 2), regional adjustments in local strains could be achieved more readily and efficiently by minor adjustments in cortical thickness by the modeling process (Goodship et al., 1979; Lanyon et al., 1982). This is an example of the modeling-remodeling “division of labor” depicted in figure 1.
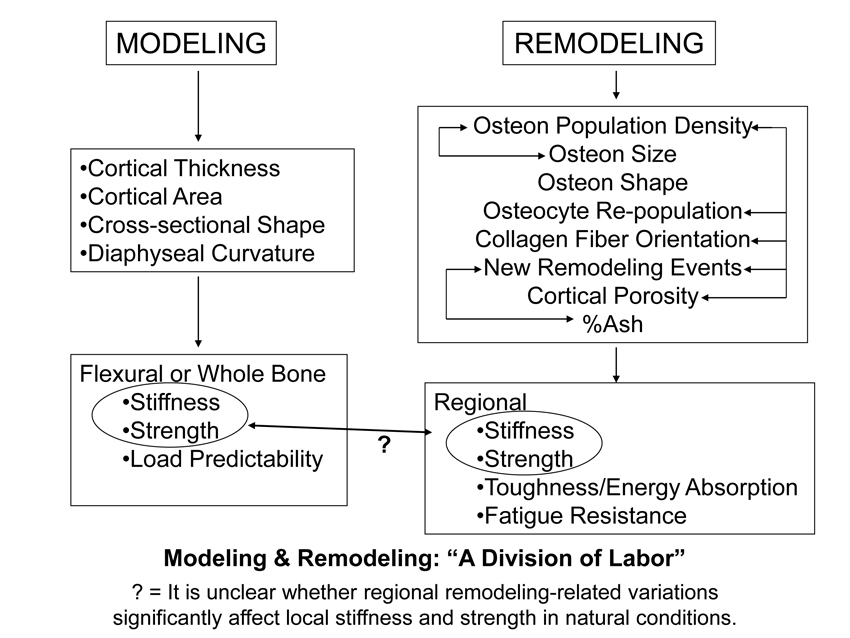
Fig. 1 Proposed bone stimulus-response algorithm. This figure demonstrates that tissue strain may not be as proximate in influencing bone adaptation as are, for example, fluid-flow dynamics and other biomechanical or biochemical stimuli. Cellular accommodation refers to the ability of bone cells to adjust to their physical and biochemical environment. Note that the various mechanisms listed in the central diamond have been proposed as candidate processes mediating bone remodeling (Currey 2002; Ehrlich and Lanyon 2002).
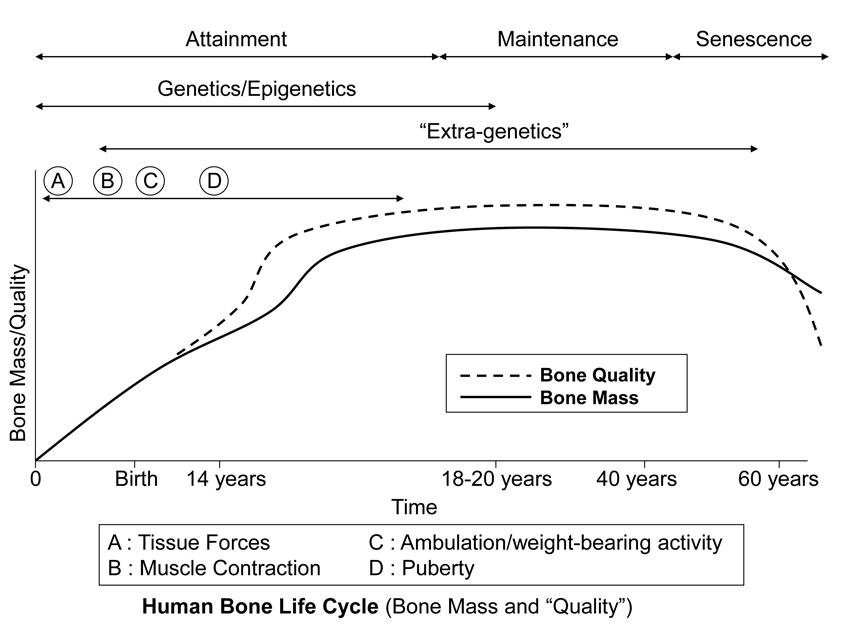
Fig. 2 This stylized depiction of ontogenetic changes in bone mass and “quality” (e.g., bone tissue mechanical properties) in humans and is adapted from several sources (Biewener and Bertram 1993; Carter and Beaupré 2001; Kassem et al. 1996). This figure helps to conceptualize the putative shifts in the temporal importance of genetic, epigenetic, and extra-genetic influences, especially with respect to varying histocompositional characteristics within or between bones. In this context, for example, the regional material variations that emerge in the “tension” and “compression” cortices during mid-to-late phases of growth of horse radii (Riggs et al. 1993) and sheep calcanei may serve to enhance the toughness and fatigue resistance for these habitual non-uniform strain environments. From the pre-natal phase and well into the attainment phase, the adaptive growth response of cartilage, or chondral modeling (not discussed in this chapter), has a profound influence on the growth and form of limb bones, especially at their epiphyses where articulations are formed with adjacent bones (Hammond et al. 2010). Shown is also the second bone mass growth spurt that occurs in humans, which has not been demonstrated in any other amniote.
If there is a division of labor between modeling and remodeling, then what are the specific “labors” of the remodeling process? To answer this it must be noted that the main mechanical properties that a bone must have, whether it is viewed as a structure or a material, are: stiffness, strength, toughness, and fatigue resistance. When regional variations in these properties are needed within the same bone (e.g., to accommodate potential strain-mode-related microdamage accumulation in bending), adequate stiffness and strength can be achieved by modeling. In contrast, local adjustments in toughness and fatigue resistance, if achieved by modeling, would likely result in an overbuilt bone lacking efficiency and safety (e.g., its mass could become too great to move it efficiently and microdamage accumulation could become highly problematic, reducing the fracture threshold) (Martin, 2003). Consequently, when regional material adjustments are needed they can be achieved by remodeling because this is the most efficient way to affect toughness and fatigue resistance, especially in local regions (e.g., between opposing cortices of the same cross-section).
Failure to recognize the modeling-remodeling distinction explains why investigators have been perplexed when they failed to detect “expected” developmental/functional adaptation in limb diaphyses in adults that experience significant differences in loading. For example, consider the results of studies by Carlson and co-workers (2006; 2008) where attempts were made to find relationships between diaphyseal shape (e.g., principal moments of inertia) and locomotor behaviors along with other non-behavioral factors in femora and humeri of free-ranging chimpanzees and other primates. The failure to detect a relationship in this solely structural context caused the authors to conclude that: “…diaphyseal shape may be unresponsive to mechanical demands of these [six] specific locomotor modes” (page 394) (Carlson et al., 2006). This supports the caution by Demes et al. (2001) (pg. 264) “against broad behavioral conclusions derived from long bone cross-sectional shape.” The modeling-remodeling ‘division of labor’ helps explain why the capacity to change diaphyseal shape is so constrained in adults, and it reminds us that modeling is relatively difficult to evoke in the skeletons of adults, but remodeling is not (Lieberman et al., 2003). The search for structural/geometric adaptation is often futile in an adult bone because the capacity to evoke adaptation via modeling is very unlikely or no longer possible — a bone’s shape appears to be highly constrained genetically/developmentally (Hansen et al., 2009; Wallace et al., 2010) unless unusual circumstances occur (e.g., increased robusticity of the racket arm when an avid tennis player is skeletally immature) (Bertram and Swartz, 1991; Haapasalo et al., 2000). In contrast, remodeling parameters, such as changes in the presence and distributions of osteon morphotypes (Skedros et al., 2009; Skedros et al., 2011; Skedros et al., 2013), would be expected to have the capacity and plasticity to adapt the bone tissue in adult bones for many of the different locomotor behaviors that were examined by Carlson and co-workers (2006; 2008).
References
Bertram JE, Swartz SM. 1991. The ‘law of bone transformation’: a case of crying Wolff? Biol Rev Camb Philos Soc 66:245-273.
Biewener AA, Bertram JEA. 1993. Mechanical loading and bone growth in vivo. In: Hall BK, editor. Bone, Volume 7, Bone Growth – B. Boca Raton, FL: CRC Press. p 1-36.
Carlson KJ, Doran-Sheehy DM, Hunt KD, Nishida T, Yamanaka A, Boesch C. 2006. Locomotor behavior and long bone morphology in individual free-ranging chimpanzees. Journal of Human Evolution 50:394-404.
Carlson KJ, Sumner DR, Morbeck ME, Nishida T, Yamanaka A, Boesch C. 2008. Role of Nonbehavioral Factors in Adjusting Long Bone Diaphyseal Structure in Free-ranging Pan troglodytes. Int J Primatol 29:1401-1420.
Carrier DR. 1983. Postnatal ontogeny of the musculo-skeletal system in the black-tailed jack rabbit (Lepus californicus). J. Zool. Lond. 201:27-55.
Carrier DR, Leon LR. 1990. Skeletal growth and function in the California gull (Larus californicus). J. Zool. Lond. 222:375-389.
Carter DH, Beaupré GS. 2001. Skeletal function and form. Mechanobiology of skeletal development, aging, and regeneration. Cambridge, UK: University of Cambridge.
Currey JD. 2002. Bones: Structure and Mechanics. Princeton, NJ: Princeton University Press.
Currey JD, Pond CM. 1989. Mechanical properties of very young bone in the axis deer (Axis axis) and humans. J. Zool. Lond. 218:59-67.
Demes B, Qin YX, Stern JT, Jr., Larson SG, Rubin CT. 2001. Patterns of strain in the macaque tibia during functional activity. Am J Phys Anthropol 116:257-265.
Ehrlich PJ, Lanyon LE. 2002. Mechanical strain and bone cell function: a review. Osteoporos Int 13:688-700.
Goodship AE, Lanyon LE, McFie H. 1979. Functional adaptation of bone to increased stress. An experimental study. J Bone Joint Surg Am 61:539-546.
Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. 2000. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27:351-357.
Hammond AS, Ning J, Ward CV, Ravosa MJ. 2010. Mammalian limb loading and chondral modeling during ontogeny. Anat Rec (Hoboken) 293:658-670.
Hansen HL, Bredbenner TL, Nicolella DP, Mahaney MC, Havill LM. 2009. Cross-sectional geometry of the femoral midshaft in baboons is heritable. Bone 45:892-897.
Heinrich RE, Ruff CB, Adamczewski JZ. 1999. Ontogenetic changes in mineralization and bone geometry in the femur of muskoxen (Ovibos moschatus). J. Zool. Lond. 247:215-223.
Jee WSS, Li XJ, Ke HZ. 1991. The skeletal adaptation to mechanical usage in the rat. Cells and Materials 1:131-142.
Kassem M, Melton LJ, Riggs BL. 1996. The Type I Type II mode for involutional osteoporosis. In: Marcus R, Feldman D, Kelsay J, editors. Osteoporosis. New York: Academic Press. p 691-702.
Kumar NC, Dantzig JA, Jasiuk IM, Robling AG, Turner CH. 2010. Numerical modeling of long bone adaptation due to mechanical loading: correlation with experiments. Annals of Biomedical Engineering.
Lanyon LE, Goodship AE, Pye CJ, MacFie JH. 1982. Mechanically adaptive bone remodeling. J Biomech 15:141-154.
Lieberman DE, Pearson OM, Polk JD, Demes B, Crompton AW. 2003. Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J Exp Biol 206:3125-3138.
Martin RB. 2003. Fatigue damage, remodeling, and the minimization of skeletal weight. J Theor Biol 220:271-276.
Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF. 1996. A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res 11:150-159.
Riggs CM, Lanyon LE, Boyde A. 1993. Functional associations between collagen fibre orientation and locomotor strain direction in cortical bone of the equine radius. Anat Embryol 187:231-238.
Skedros JG, Keenan KE, Williams TJ, Kiser CJ. 2013. Secondary osteon size and collagen/lamellar organization (“osteon morphotypes”) are not coupled, but potentially adapt independently for local strain mode or magnitude. Journal of Structural Biology 181:95-107.
Skedros JG, Kiser CJ, Keenan KE, Thomas SC. 2011. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat 218:480-499.
Skedros JG, Mendenhall SD, Kiser CJ, Winet H. 2009. Interpreting cortical bone adaptation and load history by quantifying osteon morphotypes in circularly polarized light images. Bone 44:392-403.
Tommasini SM, Nasser P, Jepsen KJ. 2007. Sexual dimorphism affects tibia size and shape but not tissue-level mechanical properties. Bone 40:498-505.
Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. 2005. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res 20:1372-1380.
Wallace IJ, Middleton KM, Lublinsky S, Kelly SA, Judex S, Garland T, Jr., Demes B. 2010. Functional significance of genetic variation underlying limb bone diaphyseal structure. Am J Phys Anthropol.

