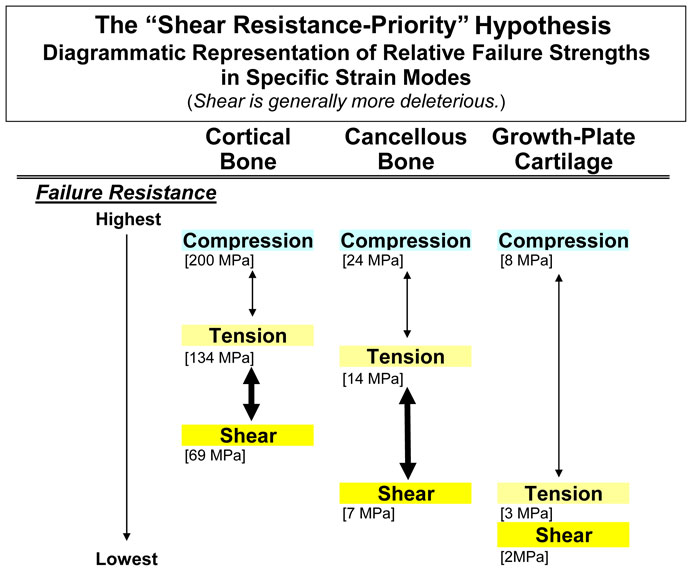
Mechanical data that support the basic premise for stating this hypothesis are diagrammatically represented in figure 1 for cortical bone, cancellous bone, and cartilage. As shown, there are three strain modes: shear, tension, and compression. In all cases, shear strains are most deleterious, with tension being a close second in most cases (Burstein et al., 1972; Reilly and Currey, 2000; Turner et al., 2001; Hiller et al., 2003; Taylor et al., 2003; Skedros and Baucom, 2007). For this reason this hypothesis could also be called the “shear/tension resistance-priority hypothesis”.
Fig. 1 Diagrammatic representation of the “shear-resistance priority hypothesis”. This shows that cortical and cancellous bone types are disproportionately weaker in shear than in tension or compression (increased vertical separation in the diagram). Although the disparity in cartilage is less marked, this tissue has poor tensile and shear strengths. This suggests that tension and shear are important in driving the ontogenetic adaptation of these tissue types. Values for this figure were obtained from these sources: Cortical bone: Cowin (1989) for human bone; values for bovine bone include: compression 197MPa, tension 130 MPa, and shear 70 MPa; Cancellous bone: Estimated from Keaveny et al. (2001) for bovine bone using strength anisotropy ratios (longitudinal ÷ transverse strength) and bone volume fractions between 0.3 and 0.5; Cartilage: The compression value is estimated from human articular cartilage (Yamada 1970). The values for tension and shear are from bovine tibia growth plates (Williams et al. 2001; Williams et al. 1999).
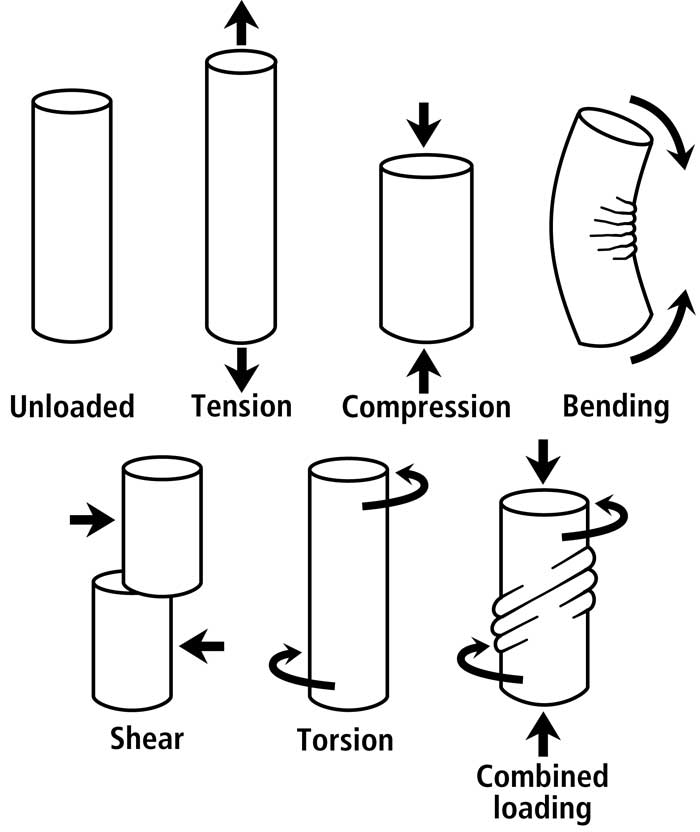
Fig. 2 Various idealized load conditions that can be imparted to a bone or bone region. Although shear would also exist in a bone subject to bending (mostly toward the neutral axis), it is more prevalent/predominant and diffusely distributed in torsion.
Non-uniform strain distributions in cortical bone, whether produced by bending or combined bending/torsion, are an essential consequence of a bone’s function (Fig. 2). A load history of habitual bending provides a good example for clarifying this point. Bending is not only a loading regime that is phylogenetically/ontogenetically highly conserved in the diaphyses of many appendicular bones (Rubin and Lanyon, 1984), but bending also produces the majority (>70%) of longitudinal strains occurring during peak loading of controlled in vivo activity of most limb bones that have been studied (Biewener et al., 1986; Biewener and Bertram, 1993)1.(see footnote). Consequently, the strain modes produced by this load regime must be accommodated. If they are not accommodated, then they will pose a significant dilemma because the “tension” and “shear” regions will be more prone to microdamage accumulation than the “compression” regions. How then, does a bone accommodate these regional disparities in the propensity to fail? As argued in Dr. Skedros’ text book chapter (Skedros, 2012),
Footnote 1. In their review article, Ruff et al. (2006) emphasize that in vivo strain data available for most bones that have been studied generally consist of a few average cyclic strain parameters that are extracted from a short period of recordings while the animal performs a very restricted task. This could be construed to mean that the concept of ‘habitual load history’ used in this chapter is excessively simplified, supporting the view that a much more complete record of strain history is required to relate bone biology and morphology to strain. But it is also important to realize that experimental studies have shown that very few loading cycles are sufficient for maintaining bone mass (Rubin et al., 1995; Rubin et al., 1996). This might suggest that even brief amounts of loading are sufficient to produce regional strain-mode-related remodeling variations between regions of the same cross-section of a limb bone diaphysis. Consequently, a “habitual” load history that produces regional variations in CFO/osteon morphotypes could, on a daily basis, be relatively brief (especially when considering the potentially deleterious consequences of shear and tension). Prospective experimental studies are needed to better define “habitual load histories” for specific bones and circumstances (e.g., (Adams et al., 1997; Fritton et al., 2000)) and how/when they are sufficient to produce differences in local histomorphology.
this cannot be adequately accomplished by the modeling process (unless the bone’s volume is sufficiently small such that microdamage formation is unlikely even when these strain-mode disparities exist). Microstructural accommodation for regional strain-mode disparities must then be accomplished by a repair mechanism — the remodeling process. One solution for the regional prevalence/predominance of tension and shear in generally exclusive regions is the formation of corresponding strain-mode-specific osteon morphotypes (e.g., a longitudinal/hooped osteon for habitual tension, and a distributed/bright osteon for compression) (Skedros et al., 2009; Skedros et al., 2011; Skedros et al., 2013).
If, however, a bone is loaded primarily in torsion, then regional variations in nanostructural/microstructural adaptations for strain modes do not occur. This is because in habitual torsion there are no significant regional disparities in strain modes. The prevalent/predominant mode in torsion is shear; by the adult stage, the adaptation for this mode is seen as relative uniformity in matrix organization (e.g., CFO patterns that are relatively more uniform across the entire bone cross-section) (Skedros and Hunt, 2004; Skedros et al., 2009). It must be emphasized that this discussion focuses on strain mode. There have been cases where relative uniformity in CFO across a bone’s mid-diaphysis has been observed even though there are locally increased concentrations of osteons in one cortical region of the cross-section. For example, this has been observed in the anterior cortex of the highly torsion-loaded sheep tibiae (Skedros et al., 2009); these locally increased osteon densities were attributed to the relatively high strain magnitudes in this complexly loaded region. There are notable examples in other bones where strain magnitude and CFO are not correlated (Mason et al., 1995; Skedros et al., 1996).
References
Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA. 1997. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J. Biomech. 30:671-678.
Biewener AA, Bertram JEA. 1993. Mechanical loading and bone growth in vivo. In: Hall BK, editor. Bone, Volume 7, Bone Growth – B. Boca Raton, FL: CRC Press. p 1-36.
Biewener AA, Swartz SM, Bertram JEA. 1986. Bone modeling during growth: Dynamic strain equilibrium in the chick tibiotarsus. Calcif Tissue Int 39:390-395.
Burstein AH, Currey JD, Frankel VH, Reilly DT. 1972. The ultimate properties of bone tissue: Effects of yielding. J. Biomech. 5:35-44.
Cowin SC. 1989. The mechanical properties of cortical bone tissue. In: Cowin SC, editor. Bone mechanics. Boca Raton, FL: CRC Press, Inc. p 97-127.
Fritton SP, McLeod KJ, Rubin CT. 2000. Quantifying the strain history of bone: Spatial uniformity and self-similarity of low-magnitude strains. J. Biomech. 33:317-325.
Hiller LP, Stover SM, Gibson VA, Gibeling JC, Prater CS, Hazelwood SJ, Yeh OC, Martin RB. 2003. Osteon pullout in the equine third metacarpal bone: Effects of ex vivo fatigue. J Orthop Res 21:481-488.
Keaveny TM, Morgan EF, Niebur GL, Yeh OC. 2001. Biomechanics of trabecular bone. Annu Rev Biomed Eng 3:307-333.
Mason MW, Skedros JG, Bloebaum RD. 1995. Evidence of strain-mode-related cortical adaptation in the diaphysis of the horse radius. Bone 17:229-237.
Reilly GC, Currey JD. 2000. The effects of damage and microcracking on the impact strength of bone. J Biomech 33:337-343.
Rubin CT, Fritton S, Sun YQ, McLeod KJ. 1996. Biomechanical parameters which stimulate bone formation: The ugly duckling of the skeletal growth factors. In: Davidovitch Z, Norton LA, editors. Biological mechanisms of tooth movement and craniofacial adaptation. Boston: Harvard Society for the Advancement of Orthodontics. p 51-59.
Rubin CT, Gross TS, McLeod KJ, Bain SD. 1995. Morphologic stages in lamellar bone formation stimulated by a potent mechanical stimulus. J Bone Miner Res 10:488-495.
Rubin CT, Lanyon LE. 1984. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol 107:321-327.
Ruff C, Holt B, Trinkaus E. 2006. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am J Phys Anthropol 129:484-498.
Skedros JG. 2012. Interpreting load history in limb-bone diaphyses: Important considerations and their biomechanical foundations. In: Crowder C, Stout S, editors. Bone Histology: An Anthropological Perspective: CRC Press. p 153-220.
Skedros JG, Baucom SL. 2007. Mathematical analysis of trabecular ‘trajectories’ in apparent trajectorial structures: the unfortunate historical emphasis on the human proximal femur. J Theor Biol 244:15-45.
Skedros JG, Hunt KJ. 2004. Does the degree of laminarity mediate site-specific differences in collagen fiber orientation in primary bone? An evaluation in the turkey ulna diaphysis. J. Anat. 205:121-134.
Skedros JG, Keenan KE, Williams TJ, Kiser CJ. 2013. Secondary osteon size and collagen/lamellar organization (“osteon morphotypes”) are not coupled, but potentially adapt independently for local strain mode or magnitude. Journal of Structural Biology 181:95-107.
Skedros JG, Kiser CJ, Keenan KE, Thomas SC. 2011. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat 218:480-499.
Skedros JG, Mason MW, Nelson MC, Bloebaum RD. 1996. Evidence of structural and material adaptation to specific strain features in cortical bone. Anat Rec 246:47-63.
Skedros JG, Mendenhall SD, Kiser CJ, Winet H. 2009. Interpreting cortical bone adaptation and load history by quantifying osteon morphotypes in circularly polarized light images. Bone 44:392-403.
Taylor D, O’Reilly P, Vallet L, Lee TC. 2003. The fatigue strength of compact bone in torsion. J Biomech 36:1103-1109.
Turner CH, Wang T, Burr DB. 2001. Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcif. Tissue Int. 69:373-378.
Williams JL, Do PD, Eick JD, Schmidt TL. 2001. Tensile properties of the physis vary with anatomic location, thickness, strain rate and age. J Orthop Res 19:1043-1048.
Williams JL, Vani JN, Eick JD, Petersen EC, Schmidt TL. 1999. Shear strength of the physis varies with anatomic location and is a function of modulus, inclination, and thickness. J Orthop Res 17:214-222.
Yamada H. 1970. Strength of biological materials. Baltimore: Williams and Wilkins Co.