The aim of adaptation is to “accommodate” not “resist” habitual/beneficial strains, at least up to a point.
While the best way to avoid fatigue failure may be to decrease microcrack incidence, Martin et al. (1998) argued that bone that is highly resistant to microdamage initiation will be inefficient at controlling microcrack propagation. The trade-off for highly stressed, fatigue-prone bone, therefore, may be efficient repair and a decrease in propagation, rather than prevention of microcrack initiation (Reilly et al., 1997). Studies have suggested that secondary osteonal bone is rather poor at minimizing microcrack formation, but rather good at attenuating the distance and rate of microcrack propagation (Burr et al., 1988; Reilly et al., 1997; Taylor, 1997; Martin et al., 1998; Reilly and Currey, 1999). From this perspective it is important to reconcile the contrary teaching of conventional wisdom that the overall goal of adaptation is to “resist” excessive bone stresses/strain. This conventional view tends to suggest that microdamage formation should be avoided altogether. An overbuilt bone then results, and the consequence could be increased microdamage anyway (please refer to section on the “volume effect”). As noted, recent empirical data do not support the conventional view. Therefore, it is best to consider adaptation as “accommodating” the safe range of strains engendered by habitual loading (Bertram and Biewener, 1988) — this is especially important when considering the benefits described below for regional variations in osteon morphotypes and predominant CFO.
The capacity of bone to accommodate/resist microdamage formation/propagation through microstructural/nanostructural modifications is called ‘toughening’. One of the most important, but non-specific, toughening mechanisms includes the introduction of microstructural interfaces such as cement lines of secondary osteons (Skedros et al., 2005; Ural and Vashishth, 2005; Gibson et al., 2006). Another important and specific, toughening mechanism has been revealed in studies that have examined thin sections of bone using polarized light. In these studies, specific osteon ‘morphotypes’ have been identified, and their increased regional prevalence can differentially/specifically toughen bone for its mechanical disparities in tension, compression, and shear (Fig. 1) (Hiller et al., 2003; Bigley et al., 2006; Skedros et al., 2009; Skedros et al., 2011a; Skedros et al., 2011b). Examples of how osteon morphotypes influence bone failure from the material level to the structural level of the whole bone are described by Ebacher et al. (2007) in their in vitro study of human tibia fractures.
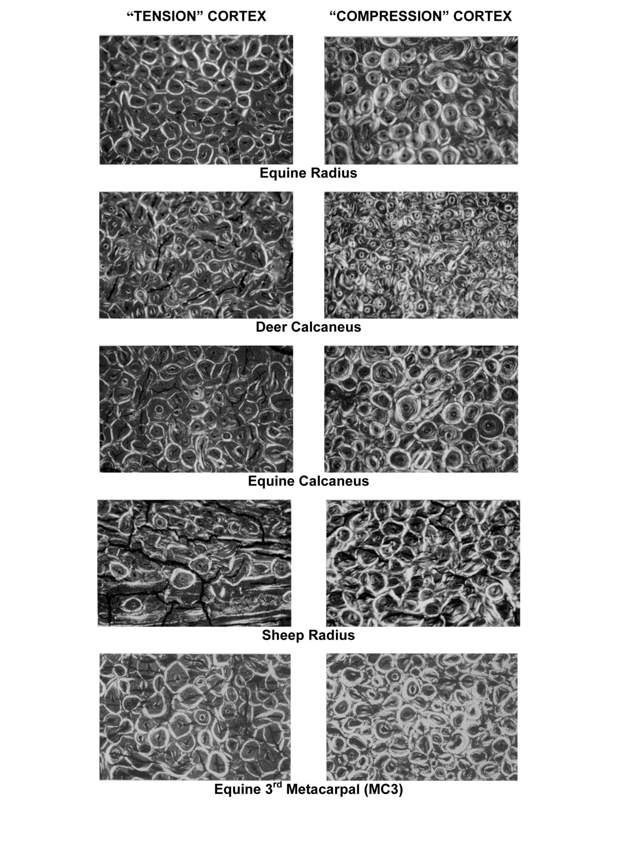
Figure 1
References
Bertram JEA, Biewener AA. 1988. Bone curvature: Sacrificing strength for load predictability? J Theor Biol 131:75-92.
Bigley RF, Griffin LV, Christensen L, Vandenbosch R. 2006. Osteonal interfacial strength and histomorphometry of equine cortical bone. J Biomech 39:1629-1640.
Burr DB, Schaffler MB, Frederickson RG. 1988. Composition of the cement line and its possible mechanical role as a local interface in human compact bone. J Biomech 21:939-945.
Ebacher V, Tang C, McKay H, Oxland TR, Guy P, Wang R. 2007. Strain redistribution and cracking behavior of human bone during bending. Bone 40:1265-1275.
Gibson VA, Stover SM, Gibeling JC, Hazelwood SJ, Martin RB. 2006. Osteonal effects on elastic modulus and fatigue life in equine bone. J Biomech 39:217-225.
Hiller LP, Stover SM, Gibson VA, Gibeling JC, Prater CS, Hazelwood SJ, Yeh OC, Martin RB. 2003. Osteon pullout in the equine third metacarpal bone: Effects of ex vivo fatigue. J Orthop Res 21:481-488.
Martin RB, Burr DB, Sharkey NA. 1998. Skeletal Tissue Mechanics. New York, NY: Springer-Verlag.
Reilly GC, Currey JD. 1999. The development of microcracking and failure in bone depends on the loading mode to which it is adapted. J Exp Biol 202:543-552.
Reilly GC, Currey JD, Goodship AE. 1997. Exercise of young thoroughbred horses increases impact strength of the third metacarpal bone. J Orthop Res 15:862-868.
Skedros JG, Holmes JL, Vajda EG, Bloebaum RD. 2005. Cement lines of secondary osteons in human bone are not mineral-deficient: new data in a historical perspective. Anat Rec A Discov Mol Cell Evol Biol 286:781-803.
Skedros JG, Kiser CJ, Keenan KE, Samuel CT. 2011a. Analysis of osteon morphotype scoring schemes for interpreting load history: evaluation in the chimpanzee femur. J Anat In review.
Skedros JG, Kiser CJ, Mendenhall SD. 2011b. A weighted osteon morphotype score out-performs regional osteon percent prevalence calculations for interpreting cortical bone adaptation. Am J Phys Anthropol 144:41-50.
Skedros JG, Mendenhall SD, Kiser CJ, Winet H. 2009. Interpreting cortical bone adaptation and load history by quantifying osteon morphotypes in circularly polarized light images. Bone 44:392-403.
Taylor D. 1997. Bone maintenance and remodeling: A control system based on fatigue damage. J Orthop Res 15:601-606.
Ural A, Vashishth D. 2005. Cohesive finite element modeling of age-related toughness loss in human cortical bone. J Biomech.

