Team Bone has examined the widely-studied turkey ulna model in the context of ultrastructure (collagen fiber orientation), microstructure, and geometry. See these studies: (Skedros et al., 2003; Skedros and Hunt, 2004) (see these papers on this website).
Implications for Functional Adaptation of Cortical Bone
Regional and age-related variations in the structural and material organization of adult mammalian limb bone diaphyses can reflect local loading history. Regional variations in matrix ultrastructural anisotropy seen as variations in predominant collagen fiber orientation (CFO) are highly correlated with regional variations in prevalent/prominent mechanical strain mode (e.g., tension, compression, shear)(Skedros, 2012). Intracortical variations in other material characteristics such as population densities of osteocyte lacunae and secondary osteons can also be important in affecting or accommodating local microdamage, mechanical strain, or matrix fluid-flow patterns. We (Skedros et al., 2003) have examined relationships between bone morphology and mechanically mediated strain/fluid-flow patterns in the turkey ulna (Qin et al., 2001). Using mid-diaphyseal transverse sections of domestic male turkey ulnae (11 sub-adults; 11 adults), we quantified developmental changes in predominant CFO, mineral content (% ash), and various microstructural parameters in cortical octants or quadrants (i.e., % ash). Geometric parameters were examined using whole mid-diaphyseal cross-sections. Rigorous studies demonstrate that this bone receives habitual bending and torsion, nonuniform matrix fluid-flow patterns, and high circumferential strain gradients along the neutral axis (cranial-caudal) region at mid diaphysis. Results showed significant porosity differences:
1) greater osteocyte lacuna densities (Of.Lc.N/B.Ar; B.Ar = bone area, including vascular porosities) (i.e., “non-vascular porosity”) in the caudal and cranial cortices in both groups
2) greater N.Lc/B.Ar in the pericortex vs. endocortex in mature bones
3) greater non-lacunar porosity (i.e., “vascular porosity”) in the endocortex vs. pericortex in mature bones
Results show that vascular and non-vascular porosities were not correlated. There were no secondary osteons in sub-adults. In adults, highest secondary osteon population densities and lowest % ash occurred in the ventral-caudal, caudal, and cranial cortices where shear strains, circumferential strain gradients, and fluid displacements are highest. Changes in thickness of the caudal cortex explained the largest proportion of the age-related increase in cranial-caudal breadth; the thickness of other cortices (dorsal, ventral, cranial) exhibited comparatively smaller changes. Only sub-adult bones exhibited CFO patterns corresponding to habitual tension (ventral) and compression (dorsal). These CFO variations may be adaptations for differential mechanical requirements in “strain-mode-specific” loading. In contrast, the more uniform oblique-to-transverse CFO patterns in adult bones may represent adaptations for shear strains produced by habitual torsional loading, which may be more prevalent in adults. These regional microstructural and ultrastructural heterogeneities may influence strain and fluid-flow dynamics, which are considered proximate signals in bone development, adaptation, and maintenance.
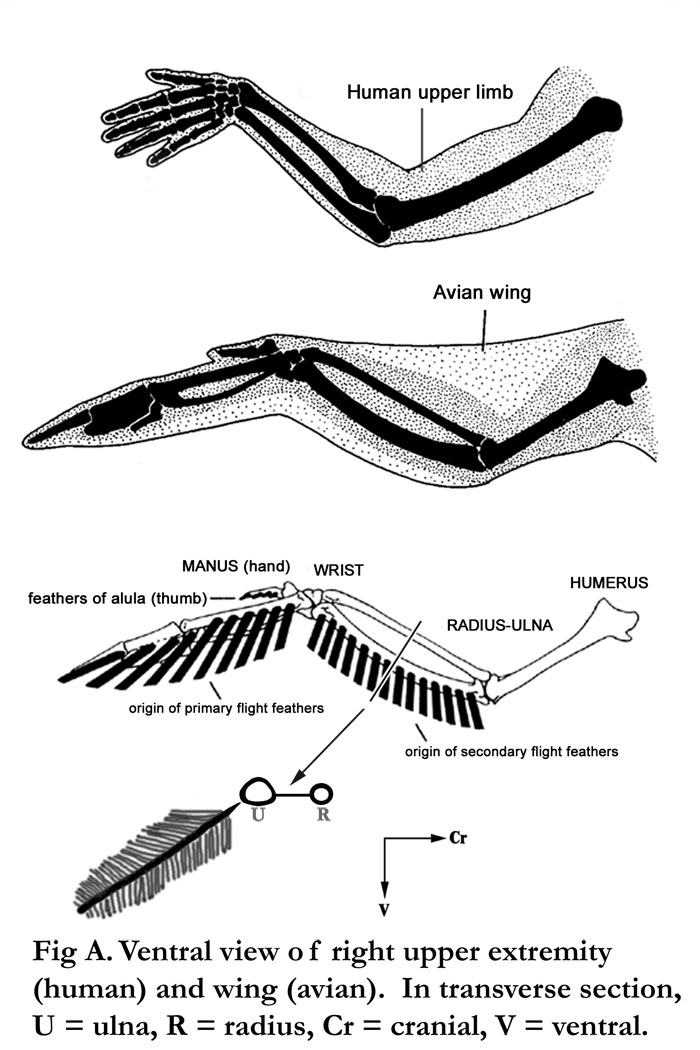
Fig. A Human vs. bird wing. Note that the secondary feathers are associated with the ulna. The primary feathers are more distal in the wing.
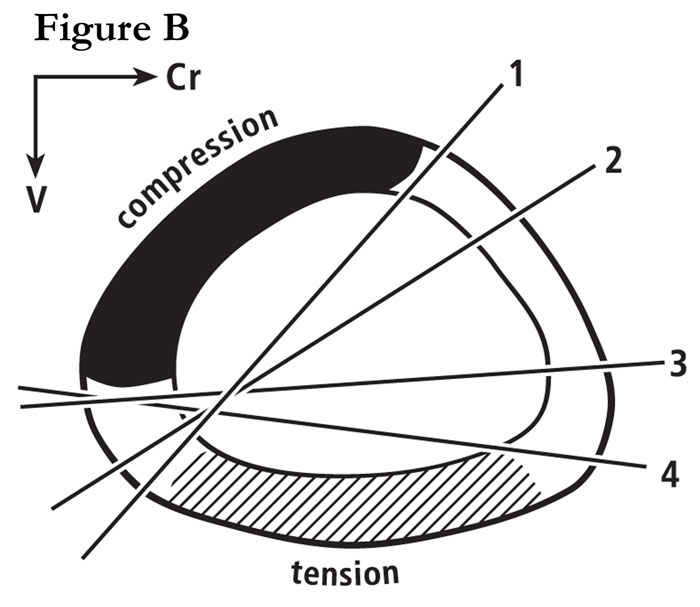
Fig. B Section through a mid-shaft turkey ulna showing the widely shifting neutral axis during wing flapping. This is re-drawn from the in vivo strain study of Rubin and Lanyon (1985). For more details, see our publication #29 on this website.
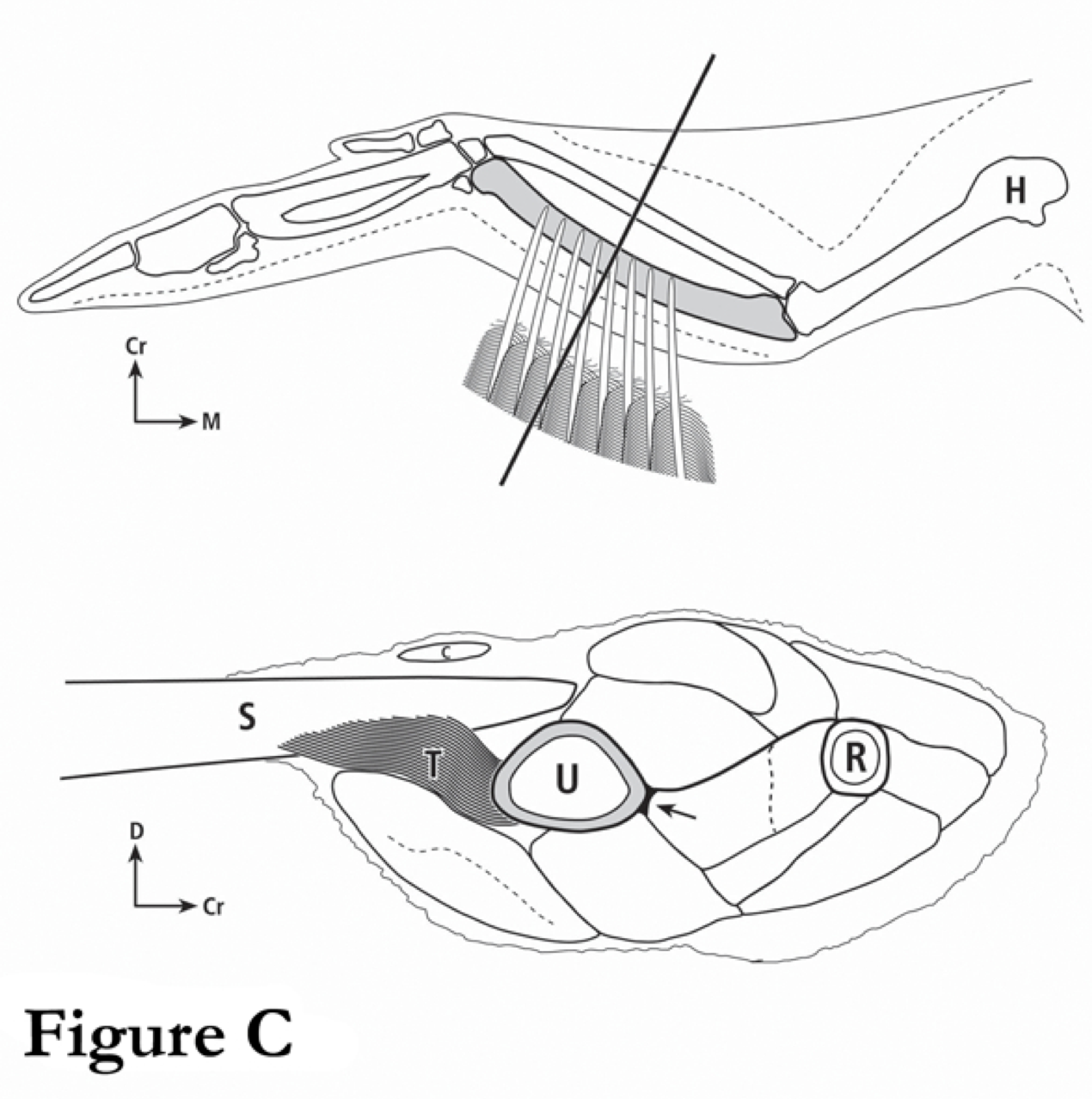
Fig. C These are drawings of a turkey wing (top) and of a cross-section through a mid-shaft turkey ulna (bottom). The muscles that are associated with the turkey ulna are considered in our study (see publication #29 by Skedros and Hunt on this website).
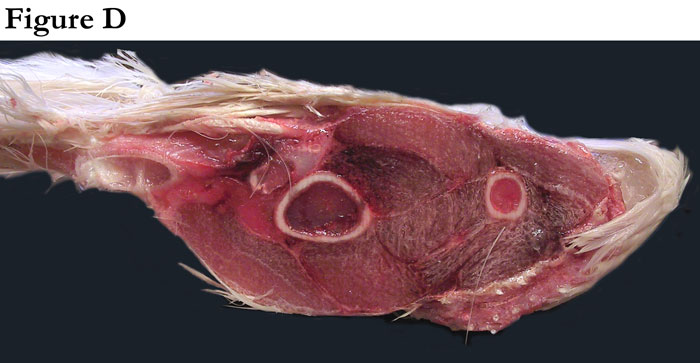
Fig. D This is a transverse cross-section of an actual of a wing of a domesticated turkey (compliments of Dr. Terry Olson, veterinarian, Moroni Turkey Hatchery, Moroni, Utah).

Fig. E Top view of dissections of a turkey wing (from part D) made by our illustrator Kerry Matz; he used these to help to ensure the accuracy of the drawings in part C.
References
Qin YX, Lin W, Rubin CT. 2001. Load-induced intracortical flow pathway and its potential role in bone adaptation. In: Bioengineering Conference: ASME.
Skedros JG. 2012. Interpreting load history in limb-bone diaphyses: Important considerations and their biomechanical foundations. In: Crowder C, Stout S, editors. Bone Histology: An Anthropological Perspective: CRC Press. p 153-220.
Skedros JG, Hunt KJ. 2004. Does the degree of laminarity mediate site-specific differences in collagen fiber orientation in primary bone? An evaluation in the turkey ulna diaphysis. J. Anat. 205:121-134.
Skedros JG, Hunt KJ, Hughes P, E., Winet H. 2003. Ontogenetic and regional morphologic variations in the turkey ulna diaphysis: Implications for functional adaptation of cortical bone. Anat. Rec. 273A:609-629.

