Cellular mechanisms of mechanotransduction in bone tissue
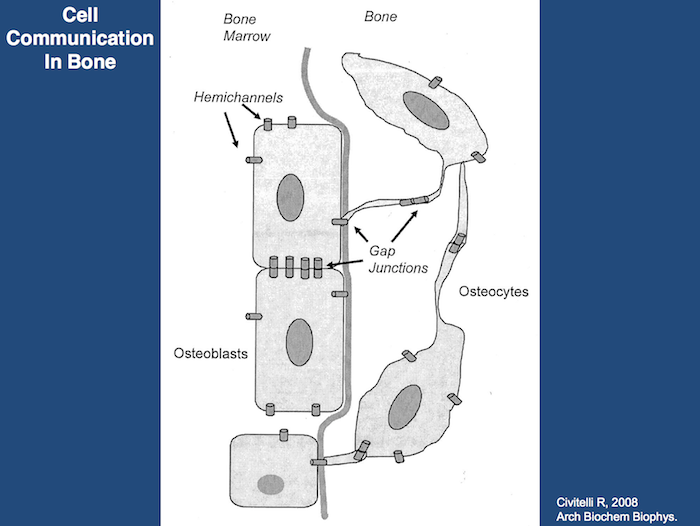
The capacity of bone tissue to alter its mass and structure in response to mechanical demands has long been recognized, but the most proximate mechanisms involved remain poorly understood. There is currently a growing consensus that the network of interconnected osteocytes and bone lining cells provides the cellular system that senses changes in bone loading and subsequently activates osteoclasts and osteoblasts to resorb or produce bone matrix. Loading produces strain (deformation) of the bone tissue, which must somehow activate the osteocytes/lining cells. Weinbaum and associates (1994) hypothesized that osteocytes are activated by the flow of interstitial fluid resulting from displacement of non-mineralized matrix in the canaliculi of strained bone. Interstitial fluid flow produces shear stress as well as streaming potentials, and their magnitude informs the osteocyte about the adequateness of the existing bone structure: normal fluid flow maintains the steady state but abnormal high flow activates the osteocyte to produce osteoblast-recruiting signals; abnormal low flow allows osteoclastic attack and bone loss. This hypothesis was tested by Burger and colleagues (1999) comparing bone’s response to fluid flow, measured as production of prostaglandins and nitric oxide (NO), by isolated osteocytes, osteoblasts and pre-osteoblasts in tissue culture. Osteocytes were by far the most mechano-responsive cells, and responsiveness increased as the bone cells matured. Fluid flow induced osteocytes and osteoblasts to express cyclo-oxygenase 2 (COX 2) (Klein-Nulend et al., 1996), an enzyme that mediates the adaptive response to loading in vivo (Forwood, 1996) and upregulated expression of endothelial-cell NO synthase (EcNOS; NO = Nitrous Oxide) (Klein-Nulend et al., 1998). These studies demonstrate the role of osteocytes as the principle mechanosensory cells of bone, and the lacuno-canalicular porosity as the structure that mediates mechanosensing.
Strain-generated flow of interstitial fluid through this porosity seems to mechanically activate osteocytes, as well as ensuring transport of cell signaling molecules and nutrient/waste products. This concept allows an explanation of local bone gain and loss, as well as remodeling in response to fatigue damage, as processes supervised by mechanosensitive osteocytes and bone lining cells (Burger and Klein-Nulend, 1999).
However, bone adaptation is more than adjustment of bone density: it also includes orientation of trabeculae and osteons along the dominant loading directions. To explain such a process at the cellular level, a mechanism must exist whereby bone remodeling leads to orientation of the secondary osteons and hemi-osteons (along the trabeculae) in the direction of the dominant loads. The mechanism that controls this process is currently unknown. Finite Element Analyses (FEA) have been developed to study if during remodeling the temporary bone deficit in the osteoclastic cutting cone will induce local stress and strain gradients that relate to local osteoclast and osteoblast activity (Smit and Burger, 2000). One of their FEA studies examined a secondary osteon in cortical bone and another examined a Howship’s lacuna in a trabecula. Both models were loaded in the longitudinal direction. Equivalent strains were determined as a measure for the deformation of the bone tissue. In both models, a field of reduced strains was found in the area of future osteoclastic attack, i.e. in front of the cutting cone of the osteon and in longitudinal direction along the surface over the trabecula. In addition, fields of elevated strain were found behind the cutting cone and at the bottom of the Howship’s lacuna, where osteoblasts are active. The authors concluded that at the tissue level, local strain distributions occur in loaded bone during both resorption and remodeling, which shows a relationship between osteoclastic and osteoblastic activity. This suggests that local strain gradients provide the information that guides osteoclasts and osteoblasts during bone remodeling. This information is sensed by the osteocyte network, as reduced or increased canalicular fluid flow. The osteocyte network then signals to the osteoclasts and osteoblasts on the bone surface, thereby recruiting them to resorb or produce bone matrix.
For additional information, please se our articles related to this topic:
1. Klein-Nulend J et al. 2012. Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton, European Cells and Materials; 24: 278-291
2. Skedros JG, Grunander TR, Hamrick MW, 2005. Spatial distribution of osteocyte lacunae in equine radii and third metacarpals: considerations for cellular communication, microdamage detection and metabolism. Cells Tissues Organs; 180: 215-236
3. Skedros JG, 2005. Osteocyte lacuna population densities in sheep, elk and horse calcanei. Cells Tissues Organs; 181: 23-37.
References
Burger EH, Klein-Nulend J. 1999. Mechanotransduction in bone — role of the lacunocanalicular network. FASEB J. 13:S101-S112.
Forwood MR. 1996. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res 11:1688-1693.
Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. 1998. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun 250:108-114.
Klein-Nulend J, Semeins CM, Burger EH. 1996. Prostaglandin mediated modulation of transforming growth factor-beta metabolism in primary mouse osteoblastic cells in vitro. J Cell Physiol 168:1-7.
Smit TH, Burger EH. 2000. Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res 15:301-307.
Weinbaum S, Cowin SC, Zeng Y. 1994. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27:339-360.