Bone is a composite tissue consisting of mineral, matrix (collagen and non-collagenous proteins), cells, and water. The mineral is hydroxyapatite, which is an analog of the naturally occurring crystalline calcium phosphate. Hydroxyapatite crystals, as distinct from geologic apatites, are very small and imperfect, containing fewer hydroxyl groups and many impurities such as carbonate, fluoride, acid phosphate, magnesium, and citrate. The small size of the crystals makes them ideally suited for their function in mineral ion homeostasis, because smaller crystals generally dissolve before larger crystals. Hydroxyapatite crystals also form in tissues that are not normally calcified; for example, in atherosclerotic plaque, soft tissues of some patients with abnormally high circulating calcium or phosphate, and articular cartilage of some patients with degenerative joint diseases (Boskey et al., 1988). These abnormal tissues generally appear distinct from bone because of the larger size of the crystals and the nature of the matrices upon which the crystals are deposited.
STRUCTURE AND FUNCTION
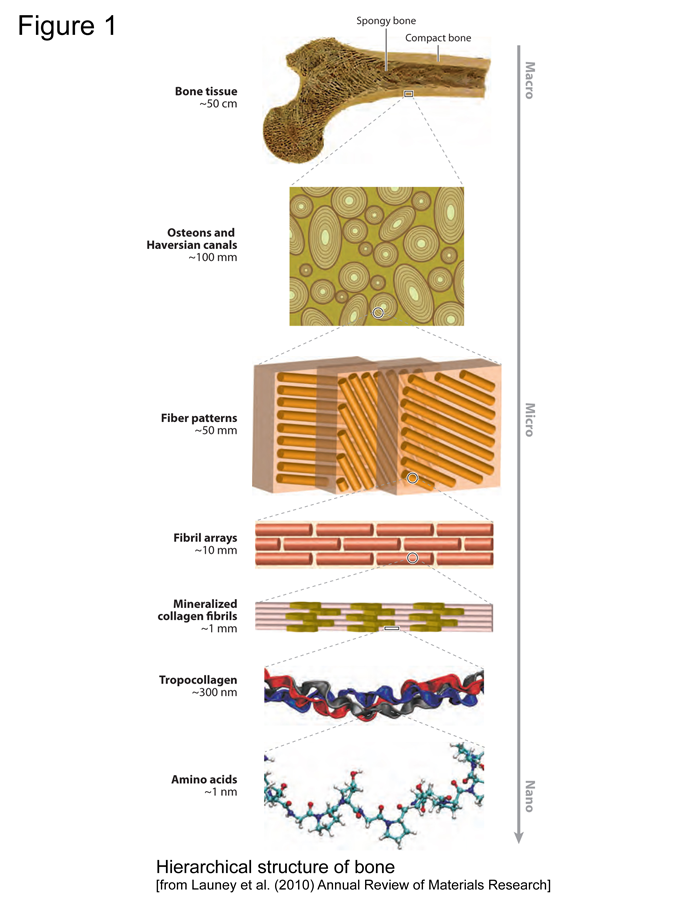
Figure 1
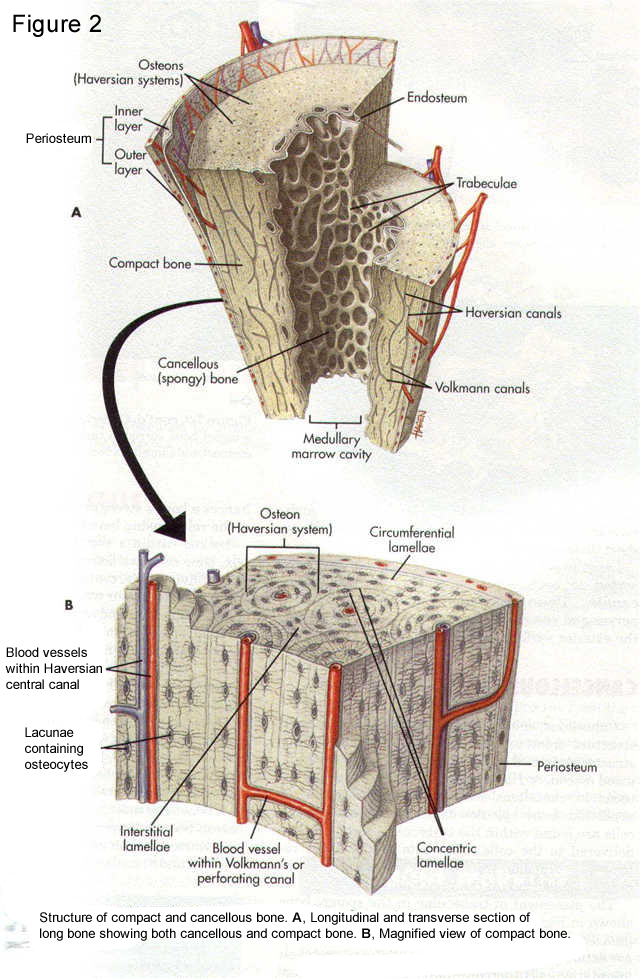
Figure 2
Bone serves as a source for calcium, magnesium, and phosphate ions and the mineral crystals in bone provide strength and rigidity for the matrix upon and within which they are deposited. The second major function of bone is mechanical; bone provides stiffness and strength in order to protect internal organs and facilitate mobility. The bone matrix is made of type I collagen, with a small fraction of non-collagenous proteins. The collagen fibrils are arranged in the extracellular matrix in patterns related to the function of the tissue in which they are found. The unique triple helical structure of collagen provides strength and flexibility to most of the connective tissues (Lees et al., 1994). The mineral crystals add extra rigidity to the collagen fibers. Collagen is stabilized by cross-links formed after translation in the extracellular matrix. The nature of these cross-links differs in the mineralized and non-mineralized connective tissues (Yamauchi et al., 1989). Analyses of the cross-links that stabilize bone collagen, as opposed to skin and tendon collagen, provide a useful marker for diseases such as osteoporosis, in which resorption of the mineralized matrix is increased (Eyre, 1992). In addition to collagen, about 5% of the extracellular matrix of bone is made up of non-collagenous proteins, including osteopontin, osteocalcin, and various bone morphogenic proteins. These proteins play crucial roles in mineral homeostasis, bone metabolism, bone formation, and bone turnover (Boskey, 1992; Roach, 1994).
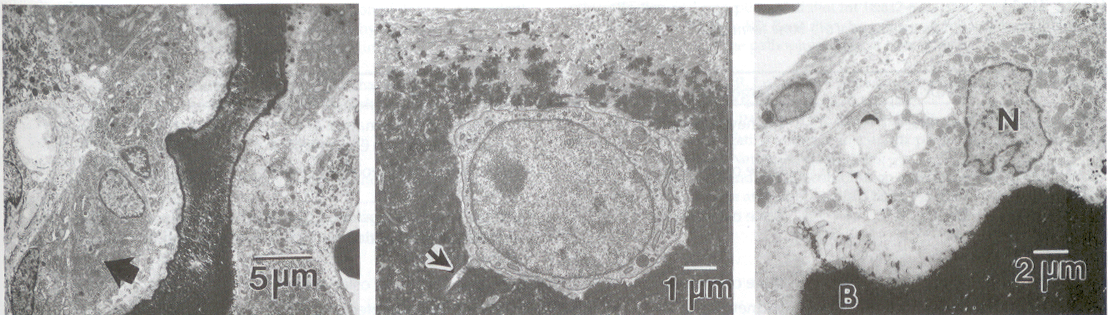
Figure 3 Osteoblasts, Center: Osteocytes, and Right: Osteoclasts in normal rat bone. Note the abundant rough endoplasmic reticulum in osteoblasts which are actively involved in bone matrix deposition. N = nuclei, B = bone surface (Boskey, 2013).
The metabolism, formation, and turnover of bone are governed by cells. Bone has four cell types: osteoblasts, osteocytes, osteoclasts, and bone-lining cells (Fig. 3). Osteoblasts and osteocytes are derived from the mesenchymal cell lineage and are closely related. Osteoblasts synthesize the bone matrix, while osteocytes, which are encased in the existing bone matrix, are more important in conveying nutrition and information throughout bone. Osteoclasts, multinucleated giant cells believed to be of macrophage origin, are responsible for removing bone. In normal tissues, the functions of osteoblasts and osteoclasts are coupled such that signals from one affect the other (Manolagas and Jilka, 1995). The distribution of these bones cells and their relative activities vary with type of bone, age, and disease state. Similarly, distributions of mineral and matrix proteins differ in various bones, and these also change with disease and age.
|
Hormones and Growth Factors Regulating Bone Formation*
|
||
|
Factor
|
Target Cells and Tissue
|
Effect
|
| Parathyroid hormone | Kidney and bone | Stimulates 1,25 Vitamin D formation and osteoclastic activity; increases circulating calcium concentrations |
| Calcitonin | Osteoclasts | Inhibits action of osteoclasts; lowers circulating calcium concentrations |
| Vitamin D (1,25D) | Osteoblasts | Stimulates collagen, osteopontin, and osteocalcin synthesis; stimulates differentiation; increases circulating calcium concentrations |
| Bone osteoclasts | Stimulates activity of osteoclasts | |
| Kidney | Stimulates calcium retention | |
| Intestine | Stimulates calcium absorption | |
| Estrogen | Bone | Stimulates formation of calcitonin receptors, inhibiting resorption |
| Prostaglandins | Osteoclasts | Stimulates resorption and formation |
| Bone morphogenetic protein | Mesenchyme | Stimulates cartilage protein and bone matrix formation; stimulates replication |
| TGF-B | Osteoblasts, chondrocytes | Stimulates differentiation |
| IL-1, IL-3, IL-6, IL-11 | Marrow, osteoclasts | Stimulate osteoclast formation |
| TNFa, GMCSF | Osteoclasts | Stimulates bone resorption |
| Leukemic inhibitory factor | 0steoblasts, osteoclasts | Stimulates osteoblast and osteoclast formation marrow |
* IL = interleukin; TGF = transforming growth factor; TNF = tumor necrosis factor; GMCSF = granulocyte-macrophage colony-stimulating factor.
BONE FORMATION (see also information on this topic in “Education Clinical” on this website)
Bone formation occurs through two distinct processes: endochondral ossification, in which bone replaces a cartilage model (anlage), and intramembranous ossification, in which bone forms directly. During embryonic development, the long bones are formed by the former process and the bones of the skull by the latter.
During endochondral ossification, mesenchymal cells differentiate into chondrocytes. These chondrocytes produce a cartilage anlage that is modified to facilitate mineralization, vascular invasion, and replacement by bone. As they mature, chondrocytes change shape and switch from making collagen found in all cartilage (II and IX) to type X collagen, a form unique to the “hypertrophic” chondrocytes at the interface of cartilage and bone. Although the precise function of type X collagen is not known, defects in its production have been found in several families with various forms of spondylepiphyseal dysplasia, a disease characterized by extreme curvature of the spine (kyphosis), implying that type X collagen has a role in endochondral bone development (Jacenko et al., 1991). Transgenic mice expressing an abnormal type X collagen develop lymphocytopenia in addition to skeletal kyphosis (Jacenko et al., 1993), indicating that type X collagen may have some other, and yet-to-be determined function in immunoregulation, in addition to preparing the calcifying cartilage matrix for bone formation.
A second alteration that occurs in the cartilaginous matrix as it matures and prepares for calcification is a modification of proteoglycan structure. Several different types of proteoglycans are present in the developing epiphyseal plate. The large chondroitin sulfate/keratan sulfate molecules (aggrecan) associate with hyaluronic acid (hyaluronan) to form high-molecular weight, space-filling molecules (aggregates) that expand to 50 times their volume. The related nonaggregating molecules found in a variety of connective tissues (versican) and a unique but related nonaggregating molecule (epiphysican) may have similar functions. In addition, there is a smaller dermatan sulfate-containing molecule with only one glycosaminoglycan chain, called decorin, which trims the surface of collagen fibrils, and biglycan, a related molecule with two component glycosaminoglycan chains per core protein. Chondroitin sulfate analogs of these dermatan sulfate proteoglycans are found in bone and tendon. The large cartilage proteoglycans (aggrecan and its aggregates and versican) function to keep the matrix hydrated and prevent calcification. The smaller proteoglycans are believed to regulate collagen fibril formation and, because of their ability to bind growth factors, play a key role in cartilage metabolism. Aggrecan and proteoglycan aggregates in solution are effective inhibitors of mineral crystal growth and formation, a finding that may explain the observation that in severe osteoarthritis, calcification occurs around chondrocytes where the proteoglycans have been degraded. As the hypertrophic cartilage is modified, mineralization commences. Initial calcification in cartilage occurs in association with both collagen and extracellular membrane-bound bodies known as matrix vesicles.
These vesicles are enriched in enzymes that facilitate both the transport of calcium and phosphate ions needed for mineralization into the vesicle and the degradation of the matrix around the vesicle. These vesicles thus provide a protected environment in which ions can accumulate and in which initial mineral deposition can occur in the absence of inhibitors. Mineral crystals break through the vesicles and may fuse with collagen-based mineral as cartilage calcification proceeds (Christoffersen and Landis, 1991).
Vascular invasion of the calcified cartilage mediated by growth factors and replacement of the underlying matrix by one containing type I collagen results in the replacement of the calcified cartilage by bone. Osteoblasts produce this bone, sequentially laying down an underlying fibrous network (consisting of fibronectin and vitronectin), type I collagen, and a variety of matrix proteins. Mineral deposition then occurs by processes described below. Once the bone is formed, it remains in a dynamic state, remodeling to provide maximum strength with minimum mass, to allow growth, and to provide a source of mineral ion homeostasis. The remodeling process (formation and resorption) is linked so that factors produced by bone-forming cells (osteoblasts) activate remodeling cells (osteoclasts) and vice versa (Manolagas and Jilka, 1995). The above table provides a partial list of the hormones and growth factors implicated in the regulation of the bone-forming and bone-resorbing cells. The receptors for many of these factors have been identified. Many of these receptors activate kinases, which in turn activate DNA-regulating proteins altering protein synthesis or enzymes that modulate matrix proteins.
References
Boskey AL. 1992. Mineral-matrix interactions in bone and cartilage. Clin Orthop Relat Res:244-274.
Boskey AL. 2013. The Composition of Bone. In: Rosen CJ, editor. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism, Eighth Edition: John Wiley & Sons, Inc. p 49-58.
Boskey AL, Bullough PG, Vigorita V, Di Carlo E. 1988. Calcium-acidic phospholipid-phosphate complexes in human hydroxyapatite-containing pathologic deposits. Am J Pathol 133:22-29.
Christoffersen J, Landis WJ. 1991. A contribution with review to the description of mineralization of bone and other calcifies tissues in vivo. Anatomical Record 230:435-490.
Eyre D. 1992. New biomarkers of bone resorption. J Clin Endocrinol Metab 74:470A-470C.
Jacenko O, LuValle PA, Olsen BR. 1993. Spondylometaphyseal dysplasia in mice carrying a dominant negative mutation in a matrix protein specific for cartilage-to-bone transition. Nature 365:56-61.
Jacenko O, Olsen BR, LuValle P. 1991. Organization and regulation of collagen genes. Crit Rev Eukaryot Gene Expr 1:327-353.
Lees S, Hanson D, Page E, Mook HA. 1994. Comparison of dosage-dependent effects of beta-aminopropionitrile, sodium fluoride, and hydrocortisone on selected physical properties of cortical bone. J Bone Miner Res 9:1377-1389.
Manolagas SC, Jilka RL. 1995. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305-311.
Roach HI. 1994. Why does bone matrix contain non-collagenous protein? The possible roles of osteocalcin, osteonectin, osteopontin, and bone sialoprotein in bone mineralisation and resorption. Cell Biology International 18:617-628.
Yamauchi M, Katz EP, Otsubo K, Teraoka K, Mechanic GL. 1989. Cross-linking and stereospecific structure of collagen in mineralized and nonmineralized skeletal tissues. Connect Tissue Res 21:159-167; discussion 168-159.

