The birth of modern day arthroplasty began in the early 1960’s when Sir John Charnley introduced cemented metal-poly-ethylene components for the hip. The original principles that he put forward with this device have yielded one of the most successful procedures in the entire canopy of medicine. Charnley’s total hip principles of 1)Resurfacing of both sides of a diarthrodial joint combined with 2)fixation of components to bone and 3) the use of materials with low wear and frictional drag, have stood the test of time to the benefit of millions of patients with degenerative joint disease.
The success and durability of the total hip arthroplasty (THA) is determined by the skill of the surgeon, the use of well designed implants and the proper patient selection. When properly applied, the long term results of THA have been excellent. Treatment of the arthritic hip joint first begins with the diagnosis of DJD, second staging of the degree of involvement, and thirdly considerations for the patients functional demands. A minority of patients exist where prudence must be exercised prior to replacing their joint because of increased risk of complications. However, THA is a very reproducible and successful treatment for a broad spectrum of patients with end stage DJD, AVN, dysplasia, fracture nonunion and other catastrophic hip lesions.
Preoperative Evaluation:
The patient’s history should be evaluated with 3 major goals in mind.
1) Determine the source of symptoms.
2) Determine whether the symptoms are severe enough to warrant consideration of THA.
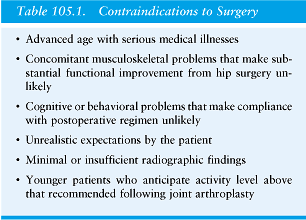
3) Determine whether the patient has contraindications to THA. (Figure 13) To address these goals, a systematic preoperative evaluation is helpful. (See periarticular pain evaluation section) This ensures the proper patient selection and exclusion as well as the collection of critical physical findings and proper imaging studies.
In addition to a full past medical history, prior history of trauma, infection, or neoplasia to the affected joint should be evaluated. As with any orthopaedic examination, first active and then passive range of motion should be assessed and documented in the hip in question as well as the contra-lateral side. Patient strength in their available range of motion especially in their hip abductors and quadriceps should be documented. The pattern of gait should also be appraised. Apparent and true leg lengths should be measured. Check and record neurovascular status. Look for evidence of local or distant infection including dental disease or lower extremity ulcers. Some patients with radiographic evidence of hip arthritis have atypical or complex symptoms that leave the source of discomfort in doubt. Special tests can be helpful in selected circumstances. A MRI scan can image early osteonecrosis of the femoral head with an occult fracture or AVN. Radio-labeled bone scans can identify stress fractures and tumors and redefine your management of the hip pain. Intra-articular injection of the hip with a local anesthetic under fluoroscopic guidance is valuable to confirm or exclude the hip as the source of pain.
Many patients with hip pain demonstrate a positive Duchenne’s sign or trundelenburg lurch, in which the patient leans toward the affected hip in the stance phase of gait. This maneuver transfers the center of gravity of the body closer to the affected hip, and reduces the resulting joint reactive force. Pain is often present at the extremes of hip motion, especially when flexion is combined with internal rotation where the maximum surface area of the femoral heads rubs across the acetabulum. The Stinchfield test, (Figure 15) where the patient performs a resisted straight-leg raise in a supine position, selectively loads the hip. It can be used to help distinguish hip joint pain from other nearby musculoskeletal sources. Intra-articular hip pain is usually reported as groin pain. Soft-tissue tenderness around the hip can result from trochanteric bursitis or local soft-tissue problems such as tendinitis. It is helpful to remember that intra-articular pain generators of the hip can be felt in the groin, buttock, lateral hip, “deep hip area,” thigh, knee, or a combination of these areas. Pain is typically exacerbated with weight bearing and prolonged ambulation. The reason why intra-articular hip pain and lumbar spine pain are often referred to the knee is because the obtorator nerve (L2,L3,L4) which innervates innervates the acetabular portion of the hip, also innervates the synovium on the medial side of the knee and thigh. (Figure 14) It is important to exclude spinal, neurologic, vascular, and tumor problems as sources of the pain. Symptom severity should be staged with pain intensity and frequency. The degree to which pain limits ambulation and functions like climbing stairs and other activities such as putting on shoes and socks, getting in and out of vehicles, and sexual function should be noted. Realistic expectations for a THA should be discussed in the context of knowing what limitations the current hip problems place on a patient’s life and whether these limitations can realistically be improved with arthroplasty.
Figure 13 (Taken from Chapman’s Operative Orthopaedics)
Figure 14
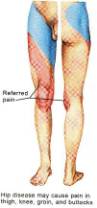
Figure 15 (Taken from Chapman’s Operative Orthopaedics) The Stinchfield test: With the patient in a supine position, a resisted straight leg raise loads the hip joint selectively. Pain in the hip, particularly in the groin or proximal or anterior thigh, with this test suggests hip joint pathology.
Radiographic Evaluation:
The standard radiographs needed for diagnosing and staging joint disease of the hip include an anteroposterior (AP) film of the pelvis, an AP film of the affected hip and upper part of the femur, and a lateral view of the hip. Frog lateral or Lowenstein lateral films provide the best lateral view of the proximal femur, but true lateral films of the hip are necessary to provide a lateral view of the acetabulum. On the AP view the patient is supine with the foot internally rotated 15º to see the femoral neck clearly. The acetabular index should be measured to determine the coverage adequacy of the superior lip of the acetabulum. This is done by drawing a line from the lateral aspect of the acetabulum down to inferior aspect of the teardrop, and measuring the angle between this line and a horizontal line. If the acetabular index is > 45º, the patient most likely has acetabular dysplasia and superior bone grafting may be necessary. True lateral views should be obtained on all patients suspected of having a hip fracture or dislocation in stead of a frog leg lateral which may displace the fracture. True lateral is obtained with the patient supine and the opposite hip is flexed and abducted with the cassette placed against the lateral aspect of the affected hip. The central beam is directed horizontally toward the groin with about 20º of cephalic tilt. Magnification of the film should be taken into consideration for templating purposes. Generally, image magnification of 20% can be expected if the X-ray tube is positioned 100 cm from the focal plane of the film or cassette. The center of the film should be directed toward the femoral head. Electronic films can produce unmagnified images or clearly define the magnification for which corresponding templates should be used.
It is vital to search for contraindications (Figure 13) for which a THA should be considered only extremely cautiously. To prevent future complications of instability or recurrent dislocations it is important to establish whether the patient has adequate neurovascular function and muscle strength to assure a stable hip endo-prosthesis. Any distant sites of infection that might elevate the risk of implant infection should be identified. Active infection is a contraindication to THA. In most circumstances anatomically distant bacterial infection should be treated before THA. Hip area pain in the absence of demonstrable joint pathology is a contraindication to surgery. (operating for “vague pain” without a diagnosis will only cause pain for you and your patient) Patients that have significant medical illness or other orthopaedic derangements that would preclude the patient from obtaining substantial functional benefit from THA have relative contraindications to surgery. Such patients include paraplegic or other non-ambulatory patients. Cognitive and behavioral problems that may preclude compliance with the postoperative regimen, such as hip dislocation precautions, are also relative contraindications to surgery. Patients with significant comorbidities should be evaluated for risk optimization by an internist. Nonoperative management is appropriate for patients with minimal damage to the hip joint and mild hip symptoms, as well as for those who by virtue of age, activity level, or medical problems are not candidates for operative treatment. The mainstays of nonoperative treatment include nonsteroidal anti-inflammatory medications, activity modification, use of a cane and unloading maneuvers, and, when appropriate, and weight loss. As with all joint replacement surgeries, these conservative alternatives should be exhausted prior to undergoing total hip arthroplasty.
INDICATIONS
An essential prerequisite for consideration of elective THA is significant hip pain, dysfunction, or both that is caused by intra-articular hip problems. This finding must be confirmed by radiographic evidence that the hip joint is damaged sufficiently to explain the symptoms. The symptom severity necessary to justify hip arthroplasty must also be weighed in light of the potential operative risks and benefits of a given patient. The more severe a patient’s pain and disability, the stronger the indication is. Most patients with pain that markedly limits ambulation, interferes with sleep, or requires narcotic pain medication are strong candidates for surgery. Patients with moderate to severe pain that occurs with moderate activity such as walking several blocks also deserve strong consideration for surgery. Severe painless loss of function due to hip stiffness, particularly if it is associated with a marked flexion contracture, is a relative indication in a few patients. The threshold for undergoing surgery should be higher in young and very active patients who are at higher risk for mechanical arthroplasty failure and subsequent multiple revision procedures.
By far the most common diagnosis leading to hip arthroplasty is osteoarthritis. Other diagnoses that confer sufficient pain and deformity to indicate THA include: inflammatory arthritis, osteonecrosis of the femoral head, developmental dysplasia of the hip (DDH), congenital hip abnormalities and traumatic conditions leading to secondary degenerative arthritis of the hip. Some proximal femoral fracture nonunions, and acute displaced proximal femur fractures are not ideal for internal fixation or hemiarthroplasty and often require THA. Many tumors around the hip require resection and reconstruction by THA. Interestingly, it is thought that as many as 48% of patients who require a total hip arthroplasty for degenerative joint disease have DDH as the underlying diagnosis. If left untreated, 25% to 50% of people affected with DDH may suffer from osteoarthritis by age 50. (26). The NIH consensus statement for total hip replacement concluded that “Total hip replacement is an option for nearly all patients with diseases of the hip that cause chronic discomfort and significant functional impairment. Most patients have an excellent prognosis for long-term improvement in symptoms and physical function.” (27)
Types of Implants:
Standard implants either employ cement or biologic interdigitation (press fit) for long term fixation of both acetabular and femoral components. A trend toward the use of noncemented fixation in THA is justified by reports of higher rates of loosening in young active patients with cemented implants. Nevertheless, the NIH consensus statement for total hip replacement concluded that “At this time, a cemented femoral component using modern cementing techniques, paired with a porous-coated (noncemented) acetabular component, can give excellent long-term results.” (27) This data showed higher rates of loosening with cemented acetabular sockets compared to porous coated noncemented sockets. However, the tendency is to use non-cemented femoral stems in young active patients where cemented stems are reported to have higher long term loosening.
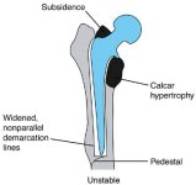
With cement fixation there is static mechanical interlock of the cement “methyl-methacrylate” to the interstices of bone. If increased cyclic loading leads to microfracture of the cement, the cement will obviously not remodel because it is not alive and the implant will ultimately loosen. However, implants with press fit or “biologic fixation” have the potential to remodel “spot welds” that can provide life long fixation after microfracture at the bone implant interface.(figure 16) Successful bone in growth requires an initial rigid fixation with micromotion of the prosthesis below 150 microns to prevent fibrous in growth. As fibrous in growth sets in, continued prosthetic micromotion and loosening cause decreased remodeling potential and ultimately pain will ensue (Figure 17). If a fibrous capsule forms around the prosthesis, it will settle and become biomechanically unstable (Figure 18). Biologic fixation is accomplished by either on growth with a grit blasted metallic surface or in growth with a porous coated surface. A porous surface allows bone to interdigitate within pores and is optimum with pore size between 50-150 microns and gaps between pores less than 50 microns. (28) The initial rigid fixation required for bony in growth into porous coated endo-prosthesis is accomplished by a technique known as “press fit”. Here the bone is prepared for a femoral or acetabular component slightly smaller than the one actually inserted (usually about 1-2mm undersized). The component is then inserted with a tight fit as the bone expands around the prosthesis generating hoop stresses that will minimize micromotion and secure the component until bone ingrowth can be accomplished. In order for stable bony ingrowth the implant must have a cortical bone seal. Cancellous bone may be able to provide ingrowth, however it can not provide the initial mechanical strength for stable implantation. (Figure 16)Therefore, the implant should be seated against stable cortical bone such as a good cortical rim of the acetabular cup for the acetabular component. Surface coating with hydroxyapatite (HA) is used as an adjuvant fixation on porous coated implants. HA has osteoconductive properties that readily receive osteoblasts leading to rapid bony ingrowth and closure of gaps between bone and prosthesis. HA coated implants can pose some problems in revision as the bone can bond so strongly to the implant to cause significant problems with implant removal.
Figure 16 Stable ingrowth of a press fit stem
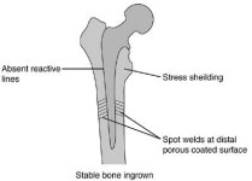
Figure 17 Fibrous ingrowth

Figure 18 Unstable stem
Optimal interdigitation of cement to bone with any endoprosthesis is accomplished with good cement technique. Several generations of cementing techniques have evolved with unresolved controversy. Newer techniques employ pressurization and vacuum preparation techniques to avoid air pores in the cement that could crack. Cement mantle defects lead to concentrated stress at the area of bone stem contact and can eventually lead to loosening. The femoral canal is also prepared with pulsatile lavage to maximize debris clearance and cement interdigitation. It is suggested that a minimum of 2mm of cement mantel surround the stem in bone. However, in larger femurs the “two-thirds” rule is often applied with two thirds of the prepared canal being replaced by the stem and the other one third by cement. If cemented femoral implants are chosen, the surgeon must decide whether to use an implant with a collar. If a prosthesis has a collar that is seated on the cut surface of the neck or if there is a transmitting layer of cement in contact with the bone and the undersurface of the collar, there will be axial loading transferred to the bone from the prosthesis. Stress transfer to the femur is desirable in THA because it provides physiologic stimulus for maintaining bone mass and preventing disuse osteoporosis from stress shielding. Although the role of a collar in preventing loosening of a cemented femoral component has not been clearly established, any loading of proximal medial neck will theoretically decrease bone resorption and thereby reduce stresses in the proximal cement because load is shared by the bone. The collar also serves as a simple means of determining depth of insertion of femoral component, since vision is temporarily obscured by extrusion of the cement;
The ideal press fit femoral stem should be minimally stiff and maximally stable, and it should prevent migration of wear particles from the metal/ poly articular interface to the distal stem. This barricade of particles reduces the “effective joint space” and prevents osteolysis from occurring at points of fixation along the stem. Previous cementless designs, which incorporated noncircumferential porous coating, demonstrated an increased incidence of distal osteolysis, occurring beyond the porous coating. However, introduction of circumferential coating in both proximally and extensively coated implants have an inherent barrier to distal particle migration. With the effective joint space minimized, the greater and lesser trochanter may be associated with the development of osteolysis but not in the meta-diaphasis where fixation is obtained.
Minimizing femoral stem stiffness may result in less thigh pain from stress concentration of forces at the stem tip. The causes of this pain are thought to be due to modulus mismatch of the stem and bone (the stem being more rigid than the bone) allowing for excessive stress transfer from stem to host femur. As many as 20% of patients with a press fit femoral prosthesis have mild pain, 11% will have moderate pain (which limits some activities), and about 2% will have severe pain. Extensively coated stems theoretically generate significant force concentration at the tip where both rigid stem and points of fixation end, concentrating forces at this point where native bone begins. This is one reason many surgeons choose proximally coated stems in primary hips in place of extensively coated stems. Nevertheless, one study comparing cementless extensively coated and proximally coated stems to cemented femoral implants, showed the highest incidence of thigh pain in proximally coated stems with similar intensity through all groups.(41) Extensively coated stems are often used in revision situations where distal fixation is required. Proximally coated stems should have a circumferential coating and bony ingrowth completely surrounding the prosthesis and prevent particulate migration. If there is minimal biologic ingrowth or spot welding to provide fixation, subsequent micro-motion at bone prosthesis interface and eventual loosening will evolve. Femoral stem position can also contribute to thigh pain as it is more common with varus stem positioning. Varus malpositioning causes the distal lateral cortex to hypertrophy as the bone attempts to remodel at this area of extreme force. Other factors include the host bone morphology and stem size and stem size mismatch.
Methods that minimize stem stiffness include creating slots like a clothespin design or creating grooves both of which increase the flexibility of the implant. However, simply increasing the flexibility of the implant with out considering the need for additional fixation is unwise. More flexible stems will allow for increased micromotion if their fixation is not augmented. Increasing fixation with out thought of its effect on load transfer could lead to stress shielding and ultimately weakly remodeled bone. The more fixation a stem has the more it transfers the entire load to the distal end of the fixation, which is the tip the stem in a extensively porous coated stem. This leads to stress shielding of the more proximal sections of bone along the stem since they “feel no load” and stress concentration is at the tip. Stems that are fully porous coated have a 2-4 fold increase in the incidence of pronounced bone resorption from stress shielding compared to proximally coated stems. (30) This is another reason many surgeons prefer proximally coated stems for primary hip replacement. Stress shielding can be seen radiographically as remodeling of proximal femoral bone, decreased proximal bone density, and thinning of the cortex, especially in the calcar region. The use of larger stems can also cause increased bone resorption from stress shielding. Stems greater than or equal to 13.5 mm in diameter showed five times the incidence of pronounced resorption compared with stems less than or equal to 12.0 mm in diameter. (30) These factors all must be balanced when choosing a prosthesis.
Management of patients with thigh pain post THA should initially be accomplished with time, since thigh pain has been shown to resolve over the first two years. Relative contra-indications to using press fit stems include: patients with “stove pipe” type femur, previous fracture, or previous osteotomy since these patients would not be expected to achieve a tight fit which is necessary for ingrowth. Also patients with poor quality bone stock are more likely to undergo plastic deformation and to allow subsidence of the femoral component. (29)
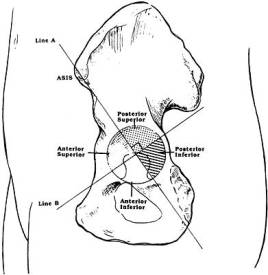
If uncemented acetabular implants are chosen, the surgeon must decide whether to augment the press-fit of the socket with further fixation such as screws. Acetabular screws should be placed in the “safe zone” of the acetabulum or posterior superior quadrant to avoid neurovascular damage. (Figure 18.5) Patients who have had preoperative radiation therapy have a higher rate of aseptic loosening from a decreased biologic ingrowth potential and are better served with cement fixation of the acetabulum.
Figure 18.5 Diagram showing the safe zone for acetabular screw placement being the posterior superior zone. The post inferior zone is often used in acetabular revisions but screws should be less than 20mm long. In the anterior two quadrants, the risk of neurovascular injury if a drill or screw extends beyond the far cortex is high. In the posterior two quadrants, there is less risk but the sciatic nerve still needs to be respected.
Materials used in implants vary significantly with the most common being cobalt chromium heads and titanium stems. Cobalt chromium has been shown to have excellent wear resistant qualities and therefore is used on the head. However, titanium has a modulus of elasticity similar to bone and therefore is used in the stem to minimize stress shielding. Materials are designed with osteolysis in mind. Osteolysis is the most common complication in total hip arthroplasty and most common cause of component failure. Osteolysis is a time dependent process which arises from inflammatory reaction against all particulate debris but chiefly polyethylene. Macrophages initiate the response by recognizing these particles as foreign and produce a host of cytokines including interleukin-1 (bone-resorbing cytokine) and tumor necrosis factor. (Figure 12) This sets off a inflammatory cascade that leads to osteoclast activation through the RANKL (receptor activated nuclear-factor kappa ligand) pathway and ultimately resorption and osteolysis. Interestingly the RANKL pathway is the final common pathway activated in all osteolytic conditions including tumors, infections and hyper-parathyroidism. New agents are currently being evaluated that selectively inhibit this pathway and might be useful in the prevention of osteolysis in artheroplasty. New materials are also now being trialed. Ceramics such as alumina have offered hope in some series of decreasing osteolysis by minimizing wear debris. Alumina ceramic provides an ultra smooth surface compared to metal on polyethylene. However catastrophic failure by fracture of the femoral head is unusually common in ceramics and a reason for caution when considering these components. With newer finishing technologies metal on metal bearing surfaces have evolved that have excellent wear characteristics. These implants do cause increased serum and urinary concentrations of metallic ions and are still experimental as well as very expensive. The recent advent of highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE) has become the most popular weight bearing surface. UHMWPE has significantly better ware rates with slightly less mechanical strength than previously used polyethylene.
Hip stability is of primary importance in planning both primary and revision surgery. Dislocation complicates between 1% and 3% of primary total hip arthroplasties (THAs) and 7% to 10% of revision procedures. Sixty percent of dislocations occur within the first 5 weeks. Closed reduction is successful in 67% of cases. If the hip keeps dislocating, revision surgery for instability is successful in only about 61% of patients. (31) The highest incidence of dislocation following primary THA is in elderly patients >80 yrs who failed femoral neck fracture fixation and convert to THA. This subgroup has dislocations reported as high as 82%. Such failure is thought to be due in part to lack of soft tissue function and inability to maintain soft tissue tensioning. Many successful techniques have been described to deal with recurrent instability including trochanteric advancement, jumbo femoral heads, modular component exchange to increase offset or leg length, bipolar or tripolar arthroplasty, and use of a constrained acetabular component. The principles of impingement and instability should always be considered when planning THA and an understanding of how to address these problems should they arise is fundamental to revision surgery.
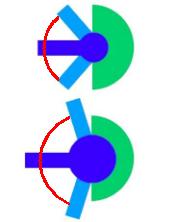
The four major contributors to hip stability in THA include 1) component design 2)component alignment 3) soft-tissue tensioning and 4) soft tissue function. The ball-cup articulation the most important design factor that determines a components stability. This articulation is often defined by its primary arc range or the amount of arc the ball can move before impinging and levering out. The primary determinant of arc is the head-neck ratio with a larger head being more stable. An increased head/ neck ratio allows for an increased arc of motion before the neck impinges on the extremes of motion (Figure 19). The center of head moves farther before it “escapes over the rim of the socket”
Figure 19. This pictorial figure demonstrates the larger arc of motion and decreased risk of impingement seen with a larger femoral head/neck ration. Two components below have the same neck diameter but the bottom figure has a larger head diameter.

A total hip prosthesis has a standard spherical head of 28 mm diameter and a cylindrical neck of 14 mm diameter. However larger heads of 36 mm can be coupled to a identical cylindrical neck as the 28 mm head and thus provides increased ROM of flexion by about 10°. This increase in ROM is valid for all joint movements, however malpositioning of the cup can affect a specific direction of motion. Conversely, a spherical head of 22 mm combined with a neck of 14 mm restricts ROM of flexion by 10° to 15°. Impingement of the femoral neck on the acetabular liner can reduce the longevity of total hip arthroplasty (THA) by inducing subluxation/ dislocation, increasing the generation polyethylene debris, and transmitting impingement forces to the fixation interface. This has been born out clinically as larger heads have been shown to have a lower dislocation rate. However, this increased stability comes at the cost of increased volumetric particulate wear. Sir John Charnley’s initial endeavors in total hip replacement made use of a femoral head that was 41. 5mm in diameter. (32) Charnley originally rationalized that the femoral head must be anatomically similar to the natural hip in order to restore the natural function of the hip. However, these initial components proved unsuccessful due to excessive wear of the bearing materials and subsequent component loosening. The recent advent of highly crosslinked ultra-high molecular weight polyethylene (UHMWPE) has allowed for the reevaluation of larger femoral head sizes. UHMWPE has demonstrated extremely low wear in vitro regardless of femoral head size and is currently being used in many implants. (33) Nevertheless particulate wear osteolysis is still the primary reason for revision. Particles can cause osteolysis anywhere they migrate within the effective joint space, which is anywhere not physically sealed off from the articulation. The effective joint space can be decreased, thus protecting portions of the arthroplasty, by using of circumferential porous coating proximally on the stem, sealing off particles from distal migration. Some of the early proximally porous-coated components had non-circumferential coating which allowed joint fluid access to the distal portion of the stem, causing distal osteolysis.
Operative Planning
Thoughtful planning of implant type and size, anatomical position, and availability before surgery reduces operative time and complications. Planning also helps the surgeon to address issues of leg length and hip stability proactively. Thorough preoperative planning therefore requires information from the patient’s history, physical examination, and radiographs. As pertaining to component alignment and soft tissue tensioning an inverse relationship has been shown between the number of THR’s performed by a surgeon and the rate of dislocation (34) Other factors that affect the potential for dislocation include preoperative diagnosis, surgical approach, postoperative management, component position, and component design.
Because the native femoral head is much larger than most prosthetic heads, good component placement is critical to hip stability. The goal acetabular component insertion is to center the primary arc range of the prosthetic in the center of the patient’s functional range. Malaligned components do not decrease primary arc range but will cause the head will suffer excessive excursion and hip dislocation at the point where the arc range intersects the range of the prosthetic. The optimal socket position is 40° to 45° of abduction on the AP radiograph and 10° to 25° of anteversion on the true lateral radiograph. To optimize risks of posterior instability most surgeons prefer to target the lower range of anteversion when an anterolateral approach to the hip is used, and the higher range when a posterolateral approach is used. This “cheating up” of the anteversion is done to provide more coverage where you need it. The anterolateral approach requires anterior surgical dislocation of the hip while the hip is posteriorly dislocated with the posterior approach.
Intraoperative trialing is important to assure that the preoperative plan of placement is optimum in all extremes of motion. Even properly placed components may still dislocate if there is inadequate soft tissue tension to hold them in place. The key soft tissue that imparts to stability is the abductor complex. Preoperative templating that asses proper head offset (Figure 20) and neck length will help restore abductor tension and hip mechanics and therefore confer stability. The femoral offset is the horizontal distance from a line drawn through the center of the femoral canal along its long axis to the center of the femoral head. Short neck lengths can cause trochanteric impingement with abduction causing the hip to lever out of its socket. Limb lengths are addressed on the AP view with 15 degrees of internal rotation. Limb discrepancies are compared from lines drawn at the level of the ischial tuberosities which intersect the lesser trochanter on each side. A horizontal line at the level of proposed neck cut should be drawn and may be used as a reference point during surgery.
It is important to maintain the patient’s original offset. (figure 20) In osteoarthritis, the acetabulum is often overgrown by osteophytes that push the femoral head laterally, superiorly, and posteriorly. In order to re-establish optimal hip mechanics, the hip center must be placed medially, inferiorly, and anteriorly. Acceptance of less femoral offset than the native hip had by more than 1 cm will result in abductor laxity and weakness. With less offset increased joint reactive forces are derived at the hip and hip instability may ensue from inability of the soft tissues to keep the head in. It is important to always know ahead of time whether the implant you are using has the option of an increased offset femoral stem as well as knowing the modularity lengthening options of the femoral head. During surgery and templating the position of the greater trochanter can be compared to the center of the femoral prosthetic head, which should coincide and give an indication of relative leg lengthening. Significant leg length discrepancies can partially be addressed with changing prosthetic offset and partially with femoral neck cut.
The socket center of rotation should be marked on preoperative radiographs. The amount of socket left uncovered laterally should be noted on the AP radiograph. On the true lateral radiograph, note the position of the socket relative to the ischium and anterior wall of the acetabulum. Knowing the expected relationship of the socket to the pelvic landmarks improves socket positioning during surgery. If the patient has normal acetabular anteverversion preoperatively, one can simply use this as a guide while positioning the reamer intra-operatively. It is important to estimate how much reaming will be necessary, and to determine whether any bone graft will be required to support the cup. The presence of protrusion or osteophyte formation may make surgical dislocation of the hip difficult. In most cases of degenerative disease, plan to remove a small amount of medial bone to place the acetabular prosthesis in an appropriate position, that is, touching the lateral aspect of the foveal bone (radiographically, the teardrop). The acetabular templates should be overlayed on the film and the appropriate socket size that matches contour of patient’s acetabulum should be selected. Care should be taken not to need to remove excessive subchondral bone to fit the socket flushly.
The neck shaft angle should be evaluated. A horizontal line at the level of the greater trochanter should intersect the center of the head of the femoral component as a measure of neck shaft angle and varus/valgus of the hip. Coxa vara, may lead to unexpected postoperative leg lengthening with possible nerve palsy as well as unexpected hip instability. In this situation consider use of a femoral stem with increased offset to balance the soft tissues and decrease impingement with less of a neck shaft angle. One radiographic pitfall is to ignore the external rotation of the femur as shown by a prominent lesser trochanter. An externally rotated femur will give the appearance that there is a relative coxa valga and also give a false impression of offset.
Fig. 20 Fem Off set
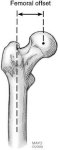
Fig.21 Cemented template
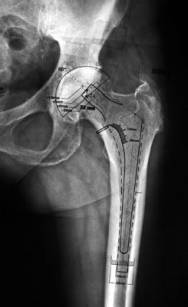
Fig 22 Extensively porous coated template
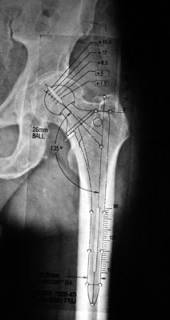
Fig 23 Proximally Coated
It is important to notice that the template in figure 21 shows a cemented femoral component with a dotted outline, which simulates the minimum cement mantle, and is also known as the broach envelope. The femoral component is outlined with a solid line. Note that the implant size chosen should leave some cancellous bone behind after broaching to provide for cement interdigitation.
Figure 22 shows an AP hip radiograph with a template for an extensively porous-coated uncemented stem. Note that with this type of implant, priority is given to getting a goodfit in the diaphysis. Figure 23 shows an AP hip radiograph template for a proximally coated uncemented stem. Note that with this type of implant, priority is given to obtaining a good fit in the metaphyseal and metaphyseal-diaphyseal junction areas.
The acetabular and femoral templates shown above mark the position of possible femoral neck lengths relative to the planned center of hip rotation. Neck lengths can be adjusted with modular necks. For example, if a given patient’s symptomatic leg was 4 mm shorter than the opposite side preoperatively, the femoral component position can be chosen to provide proper leg length with a +5 mm modular neck length. If the original anatomic femoral head center is inferior to what is marked as the templated center of the acetabulum, the limb will wind up shortened relative to the preoperative condition. Likewise, anatomic femoral head centers superior to the templated center of the acetabulum will lengthen the leg as the center of the hip is shifted downward. Also, original femoral heads with actual centers lateral to the planned center of the acetabulum reduce femoral offset relative to the preoperative status. Anatomic femoral head centers medial to the planned center of the acetabulum increase femoral offset relative to the preoperative status. The term femoral offset in this context is used as an approximation. Femoral offset classically defined refers only to the femur and is not dependent on the center of the acetabulum. To obtain proper femoral offset, which is critical in restoring soft tissue tension as well as the abductor lever arm, a modular offset femoral component can be chosen with varying lengths of offset.
Surgical Technique and Approach
There is no consensus as to the optimum surgical approach used in THA. Most approaches can be categorized as variations of the posterior approach (also known as Kocher Langenbock or Southern/Moore approaches), the anterolateral or Watson Jones approach, and the lateral or Hardinge approach. The two most popular approaches are the posterior approach and the anterolateral approach, each with their variations and pros and cons. It is generally thought that surgeons should not be rigid in their approach and technique should be based on patient profile and procedure planned.
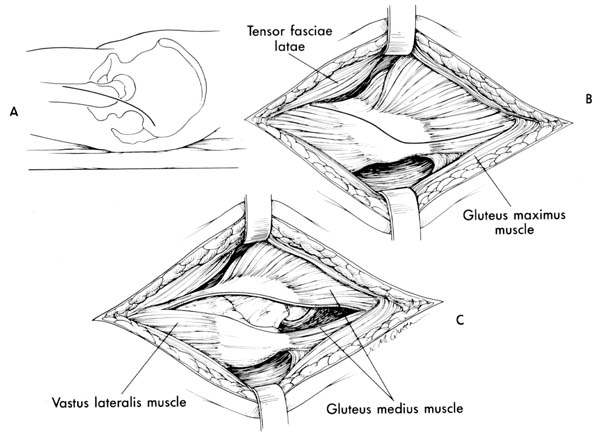
The posterior approach is advocated by many surgeons because of its ease and the superior acetabular and femoral visualization it offers. Additional benefits of the posterior approach are its preservation of gluteus medius, less postoperative limping, and no possibility of abductor muscular complications. In this approach the gluteus maximus is split and the piriformis along with the short external rotators are peeled back to expose the capsule. Because the hip is then dislocated posteriorly and the gluteus maximus is split, there is a higher dislocation rate with this approach especially in patients with neuromuscular deficits. When using this approach it is important to have a meticulous capsular and musculotendinous repair as well as consider using components with a larger head:neck ratio.
Technique: Place the patient on the unaffected side. Start the incision approximately 10 cm distal to the posterosuperior iliac spine and extend it distally and laterally parallel with the fibers of the gluteus maximus to the posterior margin of the greater trochanter. Then direct the incision distally 10 to 13 cm parallel with the femoral shaft. Expose and divide the deep fascia in line with the skin incision. By blunt dissection separate the fibers of the gluteus maximus; take care not to disturb the superior gluteal vessels in the proximal part of the exposure. Retract the proximal fibers of the gluteus maximus proximally, and expose the greater trochanter. Retract the distal fibers distally and partially divide their insertion into the linea aspera in line with the distal part of the incision. Identify the sciatic nerve by visualization or palpation. Protect it throughout the procedure. Identify the piriformis tendon and divide the fascia along its superior border. Pass a periosteal elevator through the interval between the gluteus minimus and the hip capsule. Detach the short external rotators from the posterior femur to expose the posterior hip capsule. Keep the short external rotator tendons as long as possible and tag them with a suture. Some surgeons will divide a small branch of the sacral plexus to the quadratus femoris and inferior gemellus, which contains sensory fibers to the joint capsule. Control branches of the circumflex femoral vessels on the deep border of the quadratus femoris muscle. Perform a capsular incision inferiorly and superiorly that extends from inferior to superior, then take the capsule down from the femur, leaving it attached to the posterior wall of the acetabulum. This creates a large posteriorly based capsular flap that can be repaired later. Make the capsular flap the maximum size possible. Some surgeons place a pin in the pelvis to make direct leg length measurements intraoperatively, and mark a point on the femur against which to compare measurements. The hip is then dislocated posteriorly by flexing the thigh and knee 90 degrees while internally rotating the thigh. The lesser trochanter is then identified.
Figure 24 the posterior approach
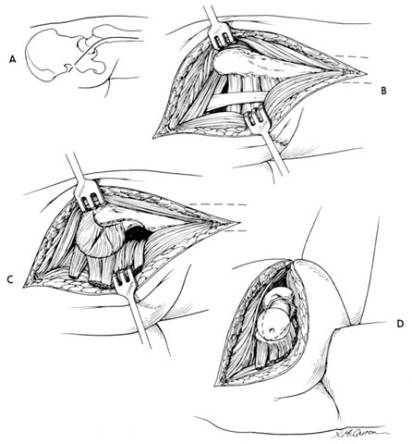
The anterolateral (Watson Jones) approach for THA has been modified with great success in the last 20 years. In this approach dissection is carried down between the tensor fascia lata and the gluteus medius. There is no true internervous plane because both of these muscles are innervated by the superior gluteal nerve. Many surgeons will dissect of the anterior ¼ of the gluteus medius to aid in visualization of the acetabulum. In this approach the hip is dislocated anteriorly and the gluteus maximus muscle is kept in tact and therefore it is associated with fewer dislocations than the posterior approach. Since the gluteus medius is affected there is some loss of abduction power and occasionally a post operative Trundelenburg limp may evolve. Consider patients at very high risk for posterior dislocation good candidates for an anterolateral approach
Technique: Begin an incision 2.5 cm distal and lateral to the anterosuperior iliac spine, and curve it distally and posteriorly over the center of the lateral aspect of the greater trochanter and lateral surface of the femoral shaft to 5 cm distal to the base of the trochanter. Locate the interval between the gluteus medius and tensor fasciae lata. The delineation of this interval is often difficult and is often palpated before it is visualized. It can be done more easily by beginning the separation midway between the anterosuperior spine and the greater trochanter, before the tensor fasciae lata blends with its fascial insertion. The coarse grain and the direction of the fibers of the gluteus medius help to distinguish them from the finer structure of the tensor fasciae lata muscle. At this point the surgeon reflects the anterior third of the gluteus medius and the gluteus minimus in continuity with the anterior third of the vastus lateralis from the proximal femur. To avoid injury to the superior gluteal nerve, take care not to extend the incision in the gluteus medius more than 5 cm from the trochanter. Note that the gluteus minimus fibers run at 90° to those of the gluteus medius. The anterior, superior, and inferior hip capsule can be excised to gain exposure or can be preserved if exposure is satisfactory. In the distal part of the incision the origin of the vastus lateralis may either be reflected distally or split longitudinally to expose the base of the trochanter and proximal part of the femoral shaft. After exposure and capsular incision the hip is then dislocated anteriorly for acetabular exposure and later instrumentation.
Figure 25 The anterolateral approach
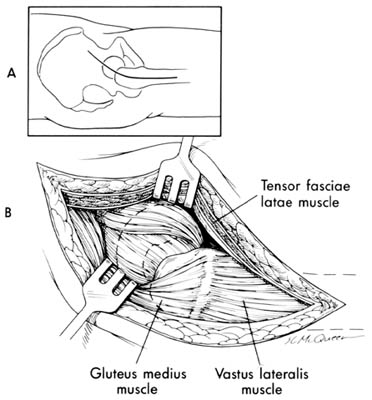
The direct lateral Hardinge approach is preferred by many surgeons. In this approach after dissecting in line through the fascia lata the gluteus medius is split in half. This plane can be carried distally over the vastus ridge and through the vastus lateralis if more exposure is needed. Because the gluteus medius is split there is often loss of abduction power and a post operative Trundelenburg limp may evolve, however this approach is associated with fewer dislocations than the posterior approach.
Technique: Place the patient supine with the greater trochanter at the edge of the table and the muscles of the buttocks freed from the edge. Make a posteriorly directed lazy-J incision centered over the greater trochanter. Divide the fascia lata in line with the skin incision and centered over the greater trochanter. It is valuable to palpate the ridge under the fascia lata that separates the bellies of the tensor fascia lata and the gluteus maximus and split the interval between the two muscles. Retract the tensor fasciae lata anteriorly and the gluteus maximus posteriorly exposing the origin of the vastus lateralis and the insertion of the gluteus medius. Incise the tendon of the gluteus medius obliquely across the greater trochanter leaving the posterior half still attached to the trochanter. Carry the incision proximally in line with the fibers of the gluteus medius at the junction of the middle and posterior thirds of the muscle. Distally, carry the incision anteriorly in line with the fibers of the vastus lateralis down to bone along the anterolateral surface of the femur. Elevate the tendinous insertions of the anterior portions of the gluteus minimus and vastus lateralis muscles. Abduction of the thigh then exposes the anterior capsule of the hip joint. Incise the capsule as desired. The hip is then dislocated anteriorly. Often cutting the base of the femoral neck facilitates acetabular exposure. During closure, repair the tendon of the gluteus medius with nonabsorbable braided sutures.
Figure 26: The direct lateral Hardinge approach
Minimal incisions (under 10 cm) are now being used for total hip arthroplasties. Some feel that in addition to cosmetic benefit minimal incisions result in less blood loss, reduced hospital stay, and faster recovery. This has not been born out clinically. Not all patients are appropriate for the minimal incision technique. Obese or very muscular patients require larger incisions for optimal viewing and component placement. Minimal incision surgery is also not appropriate in complex revision surgery. One technique for minimally invasive THA includes a standard posterolateral incision slightly posterior to trochanter; inferior to greater trochanter. A posterior trapezoidal capsulotomy and femoral head resection is performed. Custom acetabular retractors are required for exposure. An anterior capsule release is performed and a custom femoral retractor is used to elevate the femur. Capsular repair through trochanter is performed after component placement.
Despite the cosmetic appearance of minimal incisions many surgeons argue that conventional incisions offered more long-term benefit to the patient. Minimal incisions can lead to a number of complications including component malposition, failure to restore hip mechanics, and nerve injury due to blind replacement of retractors. Larger more obese patients with their thicker soft tissue envelope offer substantial difficulties to obtaining adequate exposure to the acetabulum. Long-term problems can occur from early micromotion, following minimal incisions. These techniques require a substantial learning curve and are clearly not suitable for all patients.
General Technique
Methods of implanting cemented and uncemented component are often specific to the implant design and anatomical approach used. Techniques associated with each implant differ because they have different instrumentation and because different parts of the femur are used to gain fixation. Nevertheless, several important points are common to insertion of most components beginning with preoperative positioning. Position the patient in either the lateral or supine position. The advantages of the lateral position are that the soft tissues tend to fall away from the wound, either anterior or posterior approaches are possible in this position, and the assistant has better visualization of the procedure. The main disadvantage of lateral positioning is the possibility the pelvis may tilt during surgery, making acetabular orientation difficult (Fig. 27). This problem can be reduced by making sure the pelvis is held securely with a positioning device and taking note of the acetabular orientation compared to fixed landmarks such as the corner of the room.
Figure 27
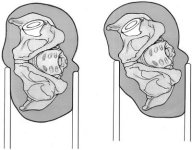
Advantages of the supine position include more certain pelvis position and easier direct comparison of leg lengths intraoperatively. The supine position restricts the surgical approach, with only anterior hip dislocation possible.
Regardless of positioning, pad the opposite leg and arms for all positions to prevent skin and nerve problems. Give the first dose of antibiotics within an hour before the skin incision is made. Perioperative antibiotics are one of the most effective means of preventing infection. Continue the prophylactic antibiotic for 24 to 48 hours after surgery.
After exposure is complete, cutting guides or implant trials can be used to mark the level and orientation of the femoral neck osteotomy. Cuts are based on preoperative planning relative to the greater trochanter, center of the femoral head, and lesser trochanter. Cut the femoral neck with an oscillating saw.
Proper acetabular preparation is crucial and it is not possible without satisfactory acetabular exposure. Poor exposure increases the likelihood of acetabular implant malposition and hip instability. Place retractors carefully around the acetabulum to avoid injury to neurovascular structures. If a posterior retractor is placed, make sure it is against bone and that the sciatic nerve is protected. During acetabular preparation sequential reamers are used to create a hemisphere of bleeding subchondral bone, but not beyond the base of the lateral aspect of the fovea. The fovea corresponds to the teardrop radiographically, and reaming usually should not be deeper than the lateral fovea. The exception to this rule applies when a shallow socket is present. It is vital to keep the reamer centered in the acetabulum to avoid eccentric reaming. A compromise should be made between excessive reaming, which removes all the subchondral bone and leaves behind only very weak bone, and insufficient reaming, which leaves a bed of only sclerotic bone. If only weak bone is left, loosening can result from implant migration through soft bone. On the other hand, if only sclerotic bone is exposed, a poor bone–cement interface may result or inadequate in growth in non-cemented components both of which can lead to early loosening. The acetabular component is inserted in same direction of reaming. The ideal socket position is 40° to 45° of abduction and 10° to 25° degrees of anteversion. With proper acetabular alignment, verified the acetabular cup should be impacted into place. A change in pitch will be audible as component seats down. If any gap is present then impact further. Many surgeons will err on the lower side of anteversion for the anterior approach and the higher side for a posterior approach.
Before preparing the femur, soft tissues just medial to the greater trochanter should be removed to allow access to the piriformis fossa starting point. If these soft tissues are not removed, there is a tendency to push instruments and the implants into a varus position. The femoral canal can be opened with a T-handled broach or awl at the piriformis fossa, which is located directly over the medullary canal. An excessively medial starting point can also lead to varus alignment. There is also a tendency when a high neck cut is made to introduce instruments into flexion due to femoral neck anteversion. This problem can be avoided by starting more posteriorly in the femoral neck.
While preparing the cemented canal, reamers should be used only to start the canal preparation to avoid excessive reaming. The broach is then used to prepare the canal in proper anteversion, usually about 15°. In cemented stems a good remaining cancellous bone layer is required for cement interdigitation. As the canal is prepared, avoid varus or valgus orientation of the instruments. A calcar planar is used after the final broach is in place to smooth the femoral neck.
Noncemented stems should obtain rigid fixation of the implant against strong, hard bone. A press-fit of the implant is predicated on having a cavity slightly smaller than the implant. In most cases, choose an implant 1 or 2 mm larger than the size of the last reamer to obtain a good press-fit. A cavity made too large from provides unsatisfactory initial implant stability and will not allow for adequate ingrowth. Too small a cavity can lead to femoral fracture when the implant is inserted.
Trial reductions to check anterior and posterior hip stability, leg length, and soft-tissue tension should always be performed. Make sure the prosthetic femoral neck does not impinge on the acetabular polyethylene. If it does, the surgeon should consider repositioning the acetabular component as prosthetic impingement can lead to hip instability or early polyethylene wear.
To close the anterolateral approach, the gluteus minimus, medius, and vastus lateralis muscles should be reattach to their beds using strong absorbable or nonabsorbable sutures passed through bone. Meticulous repair is necessary because failure of the abductors to heal can cause limp and hip instability.
To close the posterior approach, the capsule and short external rotators should be reapproximated to the posterior aspect of the abductors and the greater trochanter. The soft tissues should be attached if possible with sutures passed through bone. This may be difficult if the leg has been lengthened or if the femoral offset is markedly increased. Overtensioning the piriformis tendon should be avoided as it passes over the sciatic nerve and could compress it. A good repair of the capsule and short external rotators may reduce the risk of early hip dislocation after a posterior approach (Fig. 28) Close the remaining tissues in layers. Some surgeons prefer to use closed suction drains.
Figure 28 Posterior Approach Repair
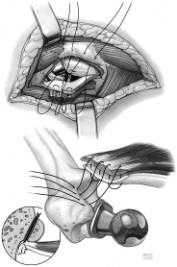
Revision Total Hip Arthroplasty
Total hip arthroplasty was originally intended for the elderly, low-demand user with significant arthritis. However, this procedure has been so successful that it is now used in increasingly younger, more active patients. With older patients living longer and arthroplasty being performed in younger patients, the need for revision arthroplasty has dramatically increased. Revision of a total hip replacement utilizes the same principles discussed in the above section. However, it is fraught with more complications and therefore is usually only indicated when mechanical failure occurs. The surgical goals of revision hip arthroplasty include 1) Removal of loose components without destroying a significant amount of host bone and tissue 2) Reconstruction of bony defects with bone graft and or metal augmentations 3)Restoration of normal hip center of rotation and 4) Placement of stable revision implants.
Once a problem has been identified on a primary hip, continued periodic follow-up is necessary to evaluate evidence of impending failure in order to intervene before a catastrophic event. Figure 29 shows a hip that is wearing eccentrically with femoral head superior laterally instead of in the center of the cup with an osteolytic lesion above the acetabulum. Of the many causes for revision including aseptic loosening, component failure, infection, and femoral fracture, the most common is aseptic loosening, which accounts for more than 80% of all failures. Septic Loosening and infection can either be obvious clinically or present as a subclinical smoldering infection that presents with component loosening and pain. Recurrent dislocation might be one of the most challenging revisions to deal with and often requires special constrained liners to keep the hip stable. Figure 31.
Figure 29
Eccentric wear
Note the head is not in the center of the
cup but has worn superiorly with a lytic cyst above.
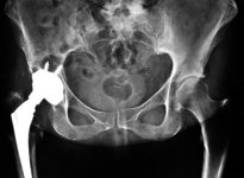
Figure 30.
Shows the multiple interfaces acting as potential particle generators in total hip replacement.
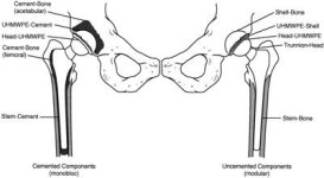
Figure 31
Bipolar constrained liner, which is inserted into a metal-backed shell. Motion is decreased with a constrained design, but stress at interfaces is increased. On the right a non-constrained and constrained liner
Revision surgery is difficult and complex with often less satisfying results than primary arthroplasty. Complications include instability/dislocation, infection, nerve palsy, cortical perforation, fracture, and DVT’s all with a higher rate than in primary replacements. Septic arthritis post THA is a potentially devastating complication and a frequent indication for revision surgery. Prosthetic infections can be divided into three categories: a) early (within 4 weeks post operatively) b) hematogenous (outside nidus like an abscessed tooth seeds the implant; if the infection persists longer than 4 weeks it is equivalent to a late infection) and c) late infections(present for more than 4 weeks). With early post-op infections, most bacteria have not formed a glycocalyx on the implant. Therefore, prior to 4 weeks an infected prosthetic can be I&D’d with modular component and poly exchange followed by 6 weeks of targeted IV antibiosis. After four weeks, a two staged re-implantation should be undertaken. Such patients have deep seeded infections that will not be eradicated with I&D alone. There is no chemical that can safely be used to remove the polysaccharide glycocalyx. In the first stage, infected components are removed, intraoperative cultures taken, and an antibiotic spacer/temporary compnent is placed. An interval of 6 weeks of targeted IV antibiosis should follow after which definitive reconstruction can take place with permanent revision components. The interval of 6 weeks is assuming a benign ESR, WBC count, CRP and clinical presentation.
In revision of the acetabulum a porous coated cup with superior screws is the preferred choice of implant. This will only work if there is an intact rim of suitable bone to provide stability to the revision press fit cup. As a general rule at least 2/3 of the rim must be intact with good bone to use a standard cup and screws. If there are significant segmental defects or decreased bone stock on the acetabular rim structural allograft will be needed supported by a reconstruction cage. (Figure 32)
Figure 32 showing an acetabular reconstruction cage with multiple screws augmenting fixation.
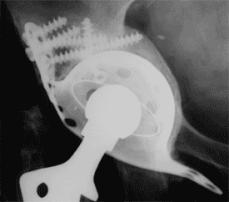
Femoral bone defects in revisions like acetabular defects are either segmental or cavitary. Significant defects can be grafted with structural allograft and cables. The most common revision stem used is an extensively porous coated (not just proximally coated) long stem prosthesis. It is important to have the revision stem pass at least 2-3 cm below the original stem to obtain fixation in the better distal bone that has not been subjected to stress shielding. (Figure 33)
Figure 33. A: Failure of a revision using a proximally porous-coated device after 2 years. B: Re-revised with a long-stemmed, fully porous-coated device with cables.
A
B 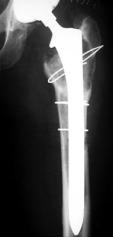
All the same principles used in primary arthroplasty should be followed in revision surgery as the goals of restoring a pain free, function hip with normal mechanics is the same in both scenarios.