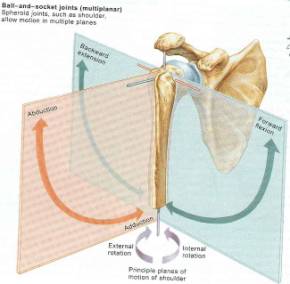
The shoulder is the most mobile joint in the body. (Figure 46) This makes arthroplasty of the shoulder joint fundamentally different than arthroplasty of the knee or hip. Nevertheless, many of the same principles of implant fixation and arthroplasty technique are very similar. The success of shoulder replacement surgery is very dependant on the surrounding soft tissues. In fact the two most important technical considerations in shoulder arthroplasty are the condition of the rotator cuff and the amount of glenoid bone stock available for resurfacing. With rotator cuff insufficiency, the humeral head will migrate superiorly and glenoid resurfacing is contraindicated. The glenoid is far less constrained than the acetabulum and yet because of its orientation the amount of sheer force it experiences is significant. This makes the glenoid especially prone to loosening and polyethylene wear. A rotator cuff deficient shoulder would direct even more sheer force towards the glenoid surface and lead to rapid wear and failure. A cuff insufficient shoulder with significant DJD is recommended to have hemi-arthroplasty with a large artificial humeral head to augment stability. Postoperatively these patients have very limited active elevation that is in the 60-80º range.
Figure 46 represents the multiple degrees of freedom that the shoulder articulation experiences.
Preoperative shoulder examination and documentation is vital to any surgical evaluation. All passive and active range of motion as well as the strength present in the available range of motion should be assessed. It is important to note the ratio of scapulothoracic to glenohumeral motion. Patients suffering from significant DJD of the shoulder reverse the normal 1:2 ratio of scapulothoracic to glenohumeral motion. This is because elevation at the glenohumeral articulation is painful compared to the scapulothoracic articulation. Nevertheless, in most patients this reversed ratio is not changed with arthroplasty. Excessive external rotation should be noted as it may indicate deficiency of the subscapularis muscle. In this case, the subscapularis can be augmented with an Achilles tendon allograft and it should be made available if this is a suspicion. Restricted external rotation may indicate severe wear of the posterior glenoid, in which case the anterior glenoid may have to be reamed to a more neutral version.
Because the nascent glenoid fossa is relatively small in size and depth, any mechanical wear or abrasion from joint destructive pathologies leave little room for resurfacing. A preoperative axillary lateral plain film or CT with axial cuts through the glenoid are crucial to evaluating glenoid wear. If the glenoid is eroded down to the coracoid process than glenoid resurfacing is contraindicated. It is also important to assess the version of the glenoid as people suffering from OA in the shoulder tend to erode the posterior glenoid resulting in relative retroversion. Glenoid retroversion should be corrected operatively to neutral version. This is most commonly accomplished with anterior reaming more than posterior bone graft augmentation. If glenoid version is not corrected, an anatomically retroverted humeral head will be unstable posteriorly. If glenoid resurfacing is undertaken in the appropriate shoulder, care is taken not to remove too much bone stock. Additionally, all-polyethylene implants approximately 4-6mm in thickness should be used. There is often insufficient bone stock to allow for a metal-backed component and the polyethylene itself. The thickness of the polyethylene insert would have to be reduced to the point that mechanical failure and cracking would be very likely. In the appropriate patient with DJD total shoulder arthroplasty has a trend toward better function and pain relief compared to hemi-arthroplasty. (48) Hemi-arthroplasty should be considered in young patients with OA, posttraumatic disorders without glenoid involvement, AVN, and massive rotator cuff tears. The approximate survival rate at 10 years is about 80%. The need for revision is significantly higher in patients who underwent hemiarthroplasty for trauma rather than for RA. Complications unique to shoulder hemiarthroplasty include erosion of the glenoid which may eventually occur in up to 70% of patients. Of patients undergoing revision hemiarthroplasty, the vast majority will be performed for a painful glenoid arthritis. In choosing a hemiarthroplasty component, it is often useful to select one with a modular head, so that the head can be removed without removing the entire stem if a future glenoid resurfacing procedure is required
Humeral stem fixation is similar in many respects to femoral fixation in the hip. Both cemented and noncemented techniques have been successful. Porous coated non-cemented implants in the humerus have a similar design rationale as those of the hip. Both stems have proximal porous coating to preserve bone stock compared to extensively coated implants that result in stress shielding and proximal bone resorption. Because the humerus is not as thick or strong as the femur, a primary press fit stem might be advantageous if it had to be revised compared to a cemented stem. A press fit stem would not require the vigorous manipulation that a cemented stem would require to remove cement while exchanging the component. In both primary and revision surgery the humeral stem should be retroverted approximately 20-30º. A cemented implant is preferred in the comminuted humeral fracture setting. Cementing provides immediate stability and augments bone that has many times already proven osteopenic.
Patient expectations should be clarified prior to surgery. Patients should not generally expect to achieve elevation above 130º. It should also be understood that improvements in function will continue for up to 18 months postoperatively. Poor prognositic indicators include: osseous deficiency of the humeral head or glenoid, non-functioning rotator cuff or deltoid, shoulder instability and previous anterior acromioplasty with excision of the coraco-acromial ligament. Patients that have had their CA ligament resected are at risk for antero-superior dislocation. If arthroplasty is contemplated in these patients the surgeon should consider reconstruction of the CA ligament and a pectoralis transfer during the arthroplasty.
Surgical Technique
The patient should be placed in the beach chair position with the torso flexed 45º and the knees flexed to 30º. The patient must lie far enough off the side of table so that the shoulder can fully extend, and be externally rotated with out interference from the table. Some adduction should also be possible. Improper positioning may complicate operative dislocation and may interfere with access to the medullary canal during broaching, and stem insertion which risks humeral fracture. A McConnel upper limb positioner or a Mayo stand can be used to hold the operative arm during the case.
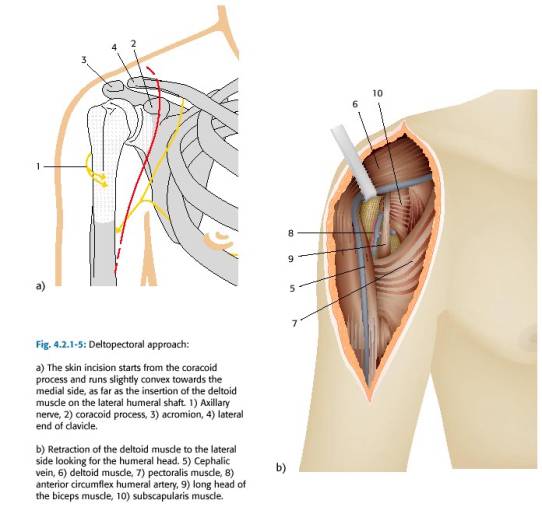
The incision begins below the middle of clavicle, passes slightly lateral to the coracoid (or directly over it), and continues distally toward upper mid portion of humerus (close to the deltoid tuberosity). Therefore the incision passes along lateral border of biceps and parallels the anterior aspect of the deltoid. The deltopectoral interval separates the plane between axillary nerve innervation to the deltoid and the lateral and medial pectoral innervation to the pectoralis major. The incision should stay just lateral to the axillary skin crease with the shoulder in neutral rotation and slight abduction. If this is not done the distal incision may be placed too far laterally. The cephalic vein helps demarcate the deltopectoral interval(Figure 47). The cephalic vein proceeds superiorly over the coracoid on its way to the subclavian vein. If the vein is not visible, look for a fat strip which may overlie it. In most cases, the vein is often retracted laterally along with the deltoid because it is usually more adherent to the deltoid. This preserves the deltoid’s venous drainage. Arterial perfusion to the deltoid is preserved because the deltoid branch of thoracoacromial artery lies parallel and lateral to cephalic vein and supplies the deltoid. The easiest way to develop the deltopectoral interval is to dissect downward just medial to the cephalic vein. The surgeon should avoid the temptation of defining the interval between the superficial muscle fibers. Often the true deltopectoral interval lies more lateral than is expected. The subdeltoid and subpectoral spaces should to be developed by blunt dissection down to their muscular insertions. The medial edge of the deltoid can occasionally be covered with clavi-pectoral fascia, in which case, it should be sharply transected, to allow entry to the sub-deltoid space. Generally the deltopectoral groove is opened distally until the insertion of the pectoralis is reached. The anterior 1/3 of the deltoid insertion may be elevated if significant posterolateral exposure is required. The cephalad 1-3 cm of the pectoralis major tendon should be incised in order to achieve better exposure of the inferior portion of the subscapularis tendon and better allow for protective palpation the axillary nerve which passes just inferior to the capsule of the subscapularis. It is useful to remember that the long head of the biceps emerges from the bicipital groove at a point just above the insertion of the pectoralis, and can be injured when the pectoralis insertion is partially incised. Once the deltopectoral interval has been fully developed, the clavipectoral fascia is exposed and is most prominent lateral to coracoid muscles. The clavipectoral fascia is differentiated from the deeper tissues, because it will not move with internal and external rotation. The tip of the coracoid (a.k.a. “the light house of the shoulder”) should be identified. It is a use landmark and it should always be remembered that axillary artery and vein as well as the brachial plexus are lie medial and inferior to the coracoid. The conjoined tendon (short head of biceps and the coraco-brachialis) should be identified coming off the tip of the coracoid. The clavipectoral fascia is then divided vertically just lateral to the conjoined tendon, up to coracoacromial ligament, exposing subscapularis tendon & lesser tuberosity. The fascia is then divided proximally at a point just lateral to the coracoid. The incision is carried distally to the level of the anterior circumflex marking the distal level of the subscapularis tendon. The musculocutaneous nerve can usually be palpated on deep surface of coraco-brachialis. The axillary nerve can be palpated inferior to the subscapularis and should be identified and protected.
Figure 47 Deltopectoral approach
The subscapularis should be fully mobilized off of the anterior glenoid to reveal the underlying joint capsule. A double spiked homan retractor is then placed on the anterior glenoid and is levered off of it to provide anterior retraction. The humeral head should then be anteriorly dislocated by extending, abducting, and externally rotating the arm. A Homan retractor can be placed behind the humeral head to maintain its dislocated position. The arm is then extended, adducted, and externally rotated and inaddition, the humerus is translated proximally (pushing up under the elbow) in order to maximize the exposure of the humeral head. The humeral head height should be noted relative to the greater tuberosity. The head usually lies slightly above the greater tuberosity. The head of the prosthesis should be superior to the greater tuberosity. This height landmark helps in the selection of humeral head component size as well as determine the proudness of the stem. At this point all peripheral osteophytes around the humeral head should be removed using a rongeur. This step must be performed carefully in order to re-establish the anatomy of the anatomical neck, which assists in correctly evaluating the nascent humeral retroversion (average is 35º). The lateral offset distance is measured from the coracoid process to the lateral most portion of the greater tuberosity. Restoring humeral offset is necessary for optimal rotator cuff function and shoulder range of motion.
At this point the humeral head is resected. This is done with the arm externally rotated about 30-40º while the saw blade is directly aligned in the AP plane of the patient. A “neutral cut” is made at the rim of the joint cartilage and should coincide with the version of the anatomic neck of the humerus. At this point the humeral canal can be prepared for either a cemented or press fit stem. The medullary canal should be broached with care taken to place lateral fin of broach slightly posterior to bicipital groove. This grove will give about 30 deg of retroversion to the prepared canal. The humeral head height should be recreated with the new implants which is about 5 mm above the greater tuberosity. While trialing the implant inferior traction should displace the prosthetic head in the glenoid about 50% inferiorly, and with no traction, the surgeon should be able to pass a finger between the acromion and the humeral head. Posterior stability can be tested with the posterior drawer test with flexion of the internally rotated arm. Anterior stability is assessed with an anterior drawer test and by externally rotating the arm while it is both adducted and abducted. If there is instability, re-assess instability with a larger humeral head. If anterior or posterior instability is present and this does not improve with increase in humeral head size, then anteversion/retroversion will have to be changed.
It is essential to obtain a tight closure of the subscapularis tendon, since anterior instability often results from avulsion of the tendon at the repair site. A more secure repair can be achieved if the subscapularis tendon can be sutured to the coracohumeral ligament (this closes down the rotator interval). Carefully note the amount of external rotation that is possible without tension after subscapularis is closed, and do not allow the patient to externally rotate beyond this point until six weeks post op.